Abstract
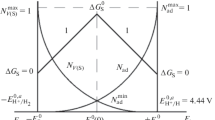
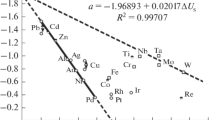
A method for the thermodynamic estimation of the active-passive transition potential of metals (Flade potential) in sulfuric acid (pH 0) is proposed. This theoretical calculation of the Flade potential (E 0 F )) differs from the conventional one in that the Gibbs formation energy of the metal oxide on the surface ΔG Ox/0Me is a sum of the Gibbs formation energy of the oxide ΔG 0 T < 0 and the surface Gibbs energy of the metal ΔG 0S,Me > 0. The technique of ΔG 0S,Me estimation used is based on the concept of the auto-adsorption of metal atoms (A) in the superficial layer and obeys the Gibbs adsorption equation σ = −GAΔμA. The estimated Flade potentials of Ni, Cr, and Fe agree with the known experimental data.
Similar content being viewed by others
References
Flade, F., Z. Phys. Chem., 1911, vol. 76, p. 513.
Frank, U.F. and Weil, K., Z. Elektrochem., 1952, vol. 56, p. 814.
Bonhoeffer, K.F. and Vetter, K.J., Z. Phys. Chem., 1950, vol. 196, p. 127. [Vetter, K.J., Elektrochemische Kinetik (Electrochemical Kinetics), Berlin: Springer, 1967]
Kolotyrkin, Ya.M., Z. Elektrochem., 1958, vol. 62, p. 664.
Sukhotin, A.M., Fizicheskaya khimiya passiviruyushchikh plenok na zheleze (Physical Chemistry of Passivating Films on Iron), Leningrad: Khimiya, 1989.
Sato, N. and Okamoto, G., J. Electrochem. Soc., 1963, vol. 110, p. 703; J. Electrochem. Soc. Japan, 1957, vol. 25, p. 199.
Vetter, K.J. and Arnold, K., Z. Elektrochem., 1962, vol. 240, p. 407.
Kolotyrkin, Ya.M., Zh. Fiz. Khim., 1960, vol. 34, p. 1138.
Zhuk, N.P., Kurs teorii korrozii i zashchity metallov (Theory of Corrosion and Metal Protection), Moscow: Metallurgia, 1976.
Wickes, C.E. and Block F.E., Thermodynamic Properties of 65 Elements — Their Oxides, Halides, Carbides, and Nitrides, Bureau of Mines Bulletin 605, Washington: US Government Printing Office, 1963.
Kaesche, H., Die Korrosion der Metalle (Corrosion of Metals), Berlin: Springer, 1979.
Zhukhovitskii, A.A., Zh. Fiz. Khim., 1944, vol. 18, nos. 5–6, p. 214.
Overbury, S.H., Bertrand, PA., and Somorjui, G.A., Chem. Rev., 1975, p. 547.
Andreev, Yu.Ya., Zh. Fiz. Khim., 1998, vol. 72, p. 529.
Andreev, Yu.Ya., Electrochim. Acta, 1998, vol. 43, p. 2627.
Andreev, Yu.Ya., Zh. Fiz. Khim., 2000, vol. 74, p. 513.
Andreev, Yu.Ya. and Kutyrev, A.E., Zh. Fiz. Khim., 2001, vol. 75, p. 689.
Andreev, Yu.Ya., Zh. Fiz. Khim., 2005, vol. 79, p. 239.
Roberts, M.W. and MacKee, C.S., Chemistry of the Metal-Gas Interface, Oxford: Clarendon, 1978.
Kubaschevski, O. and Alcock, C.B., Metallurgical Thermochemistry, Oxford: Pergamon, 1979.
Kumikov, V.K. and Khokonov, Kh.B., J. Appl. Phys., 1983, vol. 54, p. 1346.
Vitos, L., Ruban, A.V., Skriver, H.L., and Kollar, J., Surf. Sci., 1998, vol. 411, p. 186.
Foley, C.L., Kruger, J., and Bechtold, C.J., J. Electrochem. Soc., 1967, vol. 114, p. 993.
Shvabe, K., Zashch. Met., 1966, vol. 2, p. 393.
Kabanov, B.N., Elektrokhimiya metallov i adsorbtsiya (Electrochemistry of Metals and Adsorption), Moscow: Nauka, 1966.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © Yu.Ya. Andreev, 2009, published in Fizikokhimiya Poverkhnosti i Zashchita Materialov, 2009, Vol. 45, No. 6, pp. 587–592.
Rights and permissions
About this article
Cite this article
Andreev, Y.Y. Thermodynamic calculation of flade potential of Fe, Ni, and Cr taking into account surface energy. Prot Met Phys Chem Surf 45, 669–674 (2009). https://doi.org/10.1134/S2070205109060057
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205109060057