Abstract
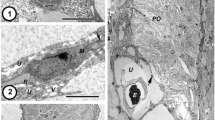
The ultrastructure of the reticular uterus has been analyzed in pregravid and gravid proglottids of cyclophillid cestodes dwelling in water (Alcataenia dominicana and A. larina) and in terrestrial hosts (Arostrilepis tenuicirrosa). Cells of the medullary parenchyma surrounding the uterus are filled with lipid inclusions in all species investigated. The hypertrophic development of small excretory ducts that surround the uterus, contact the uterine epithelium, and penetrate the diverticula is characteristic of Alcataenia dominicana and Arostrilepis tenuicirrosa. A comparative analysis of the results and the data available for other cestode species allow for the assumption that the reticulate structure of the uterus, lipid accumulation, and contacts between the uterine epithelium and the excretory ducts are morphological and functional adaptations that enable matrotrophy and the attainment of maximal fecundity by cyclophillid cestodes.
Similar content being viewed by others
References
Vinogradov, G.A., Davydov, V.G., and Kuperman, B.I., Morphophysiological characteristics of water–salt metabolism in some pseudophyllidean cestodes, Parazitologiya, 1982, vol. 16, no. 3, pp. 188–193.
Gulyaev, V.D. and Kornienko, S.A., The causes and mechanisms of emergence of miniature polymer Hymenolepididae (Cyclophyllidea, Cestoda), parasites of shrews, Tr. Zool. Inst. RAN, 2009, vol. 313, no. 3, pp. 249–256.
Korneva, Zh.V. and Kornienko, S.A., Interaction of the uterus and developing eggs in cyclophyllidean cestodes with different fecundity, Biol. Bull. (Moscow), 2014, vol. 41, no. 1, pp. 130–148.
Korneva, Zh.V., Kornienko, S.A., and Gulyaev, V.D., Morphological and ultrastructural changes in the uterus and the formation syncapsules in ontogenesis of Ditestolepis diaphana (Cestoda: Cyclophyllidea), Zool. Zh., 2010, vol. 89, no. 10, pp. 1181–1189.
Kuperman, B.I., Funktsional’naya morfologiya nizshikh tsestod (Functional Morphology of Lower Cestodes), Leningrad: Nauka, 1988.
Nazimova, D.I., Ponomarev, E.I., Stepanov, N.V., and Fedotova, E.V., Black coniferous forests in the south of the Krasnoyarsk Krai and the problems of their review mapping, Lesovedenie, 2005, no. 1, pp. 12–18.
Bryant, C., The utilization the carbon dioxide by Moniezia expansa: aspect of metabolic regulation, in Comparative Biochemistry of Parasites, London: Acad. Press, 1972, pp. 49–80.
Chomicz, L., Comparative ultrastructural studies on uterus-egg interrelations in some species of hymenolepidids (Cestoda) with aquatic life cycles, Acta Parasitol., 1996, vol. 41, pp. 191–198.
Chomicz, L. and Czubaj, A., Transmission electron micrograph studies of developing oncospheral envelopes of Fimbriaria fasciolaris (Hymenolepididae), Parasitol. Res., 1991, vol. 77, pp. 503–508.
Chomicz, L., Grytner-Ziecina, B., and Walski, M., Morphological studies on envelopes of oncospheres of the hymenolepidid cestode, Fimbriaria fasciolaris (Pallas, 1781), Acta Parasitol., 1995, vol. 40, no. 1, pp. 26–30.
Chomicz, L. and Swiderski, Z., Functional ultrastructure of the oncospheral envelopes of twelve hymenolepidid species with aquatic life cycles, Acta Parasitol., 2004, vol. 49, no. 3, pp. 177–181.
Chowdhury, N. and De Rycke, P.H., Qualitative distribution of neutral lipids and phospholipids in Hymenolepis microstoma from the cysticercoid to the egg producing adult, Z. Parasitenkd., 1976, vol. 50, pp. 151–160.
Conn, D.B., Ultrastructure of the gravid uterus of Hymenolepis diminuta (Platyhelminthes: Cestoda), J. Parasitol., 1993, vol. 79, no. 4, pp. 583–590.
Conn, D.B. and Etges, F.J., Fine structure and histochemistry of the parenchyma and uterine egg capsules of Oochoristica anolis (Cestoda: Linstowiidae), Z. Parasitenkd., 1984, vol. 70, no. 6, pp. 769–779.
Conn, D.B. and Forman, L.A., Morphology and fine structure of the gravid uterus of three hymenolepidid tapeworm species (Platyhelminthes: Cestoda), Invert. Reprod. Develop., 1993, vol. 23, nos. 2–3, pp. 95–103.
Davydov, V.G. and Korneva, J.V., Differentiation and structure of a uterus for Nippotaenia mogurndae Yamaguti et Miato, 1940 (Cestoda: Nippotaeniidea), Helmintologia, 2000, vol. 37, no. 2, pp. 77–82.
Hamilton, J.D., Dawson, A.M., and Webb, J.P.W., Observation upon smallgut “mucosal” pO2 and pCO2 in anesthetized dogs, Gastroenterology, 1968, vol. 55, pp. 52–60.
King, J.W. and Lumsden, R.D., Cytological aspects of lipid assimilation by cestodes. Incorporation of linoleic acid into the parenchyma and eggs of Hymenolepis diminuta, J. Parasit., 1969, vol. 55, pp. 250–260.
Korneva, J.V., Kornienko, S.A., and Jones, M.K., Fine structure of the uteri in two hymenolepidid tapeworm Skrjabinacanthus diplocoronatus and Urocystis prolifer (Cestoda: Cyclophyllidea) parasitic in shrews that display different fecundity of the strobilae, Parasitol. Res., 2012, vol. 111, no. 4, pp. 1523–1530.
Korneva, J.V., Kornienko, S.A., Kuklin, V.V., et al., Relationships between uterus and eggs in cestodes from different taxa, as revealed by scanning electron microscopy, Parasitol. Res., 2014, vol. 113, pp. 425–432.
Makarikov, A.A., Gulyaev, V.D., and Kontrimavichus, V.L., A redescription of Arostrilepis horrida (Linstow, 1901) and descriptions of two new species from Palaearctic microtine rodents, Arostrilepis macrocirrosa sp. n. and A. tenuicirrosa sp. n. (Cestoda: Hymenolepididae), Folia Parasitol., 2011, vol. 58, no. 2, pp. 108–120.
Mayberry, L.F. and Tibbits, F.D., Hymenolepis diminuta (order Cyclophyllidea): histochemical localization of glycogen, neutral lipid, and alkaline phosphatase in developing worms, Z. Parasitenkd., 1972, vol. 38, pp. 66–76.
Mettrick, D.F. and Podesta, R.B., Ecological and physiological aspects of helminth-host interactions in the mammalian gastrointestinal canal, Adv. Parasitol., 1974, vol. 12, pp. 183–278.
Ostrovsky, A.N., Lidgard, S., Gordon, D.P., et al., Matrotrophy and placentation in invertebrates: a new paradigm, Biol. Rev., 2015. Camb. Philos. Soc. doi 10.1111/brv.12189
Overturf, M. and Dryer, R.L., Lipid metabolism in the adult cestode Hymenolepis diminuta, Comp. Biochem. Physiol., 1968, vol. 27, pp. 145–175.
Podesta, R.B. and Mettrick, D.F., Components of glucose transport in the host parasite system Hymenolepis diminuta (Cestoda) and the rat intestine, Can. J. Physiol. Pharm., 1974, vol. 52, pp. 183–197.
Rybicka, K., Embryogenesis in cestodes, Adv. Parasitol., 1966, vol. 20, pp. 759–767.
Shimazu, T., Some cestodes and acanthocephalan larvae from euphausiid crustaceans collected in northern North Pacific Ocean, Bull. Jpn. Soc. Sci. Fish., 1975, vol. 41, pp. 813–821.
Swiderski, Z. and Xylander, W.E.R., Vitellocytes and vitellogenesis in cestodes in relation to embryonic development, egg production and life cycle, Int. J. Parasitol., 2000, vol. 30, pp. 805–817.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © J.V. Korneva, V.V. Kuklin, S.A. Kornienko, 2016, published in Biologiya Vnutrennykh Vod, 2016, No. 3, pp. 21–28.
Rights and permissions
About this article
Cite this article
Korneva, J.V., Kuklin, V.V. & Kornienko, S.A. Ultrastructure of the reticulate uterus and specific features of matrotrophy in three species of higher cestodes (Cestoda, Cyclophyllidea). Inland Water Biol 9, 234–241 (2016). https://doi.org/10.1134/S1995082916030111
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995082916030111