Abstract
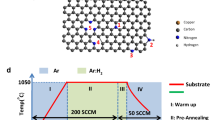
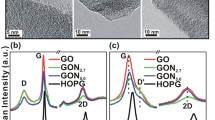
Carbon nanomaterials doped with heteroatoms, in particular, nitrogen atoms, are of great interest for electrochemical power engineering as nonmetallic catalysts or carriers of catalytically active metal nanoparticles. A nanostructured, reduced graphene oxide modified with nitrogen in a gas discharge plasma in a vacuum chamber of a magnetron-ion sputtering facility is considered. It is shown that plasma treatment of reduced graphene oxide does not cause undesirable morphological changes in the structure of carbon nanomaterial, but it leads to the incorporation of nitrogen atoms into the structure of reduced graphene oxide with the formation of pyridine-, pyrrole-, and graphite-like configurations. The application of pulsed negative bias voltages of various magnitudes to the substrate with the sample increases the concentration of nitrogen atoms to 2.6 at % and also promotes an increase in the proportion of nitrogen atoms in the pyridine form and a slight decrease in the proportion of atoms in the pyrrole form. The results allow for considering the obtained carbon nanomaterials for use as components of electrochemical devices, for example, fuel cells, in the future.



Similar content being viewed by others
REFERENCES
Z.-L. Xu, J.-K. Kim, and K. Kang, Nano Today 19, 84 (2018). https://doi.org/10.1016/j.nantod.2018.02.006
T. Chen and L. Dai, Mater. Today. 16, 272 (2013). https://doi.org/10.1016/j.mattod.2013.07.002
E. Antolini, Appl. Catal. B 88, 1 (2009). https://doi.org/10.1016/j.apcatb.2008.09.030
I. V. Pushkareva, A. S. Pushkarev, S. A. Grigoriev, E. K. Lyutikova, S. V. Akel’kina, M. A. Osina, E. P. Slavcheva, and V. N. Fateev, Russ. J. Appl. Chem. 89, 2109 (2016).
P. Trogadas, T. F. Fuller, and P. Strasser, Carbon 75, 5 (2014). https://doi.org/10.1016/j.carbon.2014.04.005
L. Du, Y. Shao, J. Sun, et al., Nano Energy 29, 314 (2016). https://doi.org/10.1016/j.nanoen.2016.03.016
D. D. Spasov, N. A. Ivanova, A. S. Pushkarev, et al., Catalysts 9, 803 (2019). https://doi.org/10.3390/catal9100803
I. E. Baranov, V. I. Porembskii, E. K. Lyutikova, et al., Chem. Probl. 17, 489 (2019). https://doi.org/10.32737/2221-8688-2019-4-489-499
S. V. Tkachev, E. Y. Buslaeva, A. V. Naumkin, et al., Inorg. Mater. 48, 796 (2012). https://doi.org/10.1134/S0020168512080158
J. W. Chiou, S. C. Ray, S. I. Peng, et al., J. Phys. Chem. C 116, 16251 (2012). https://doi.org/10.1021/jp303465u
A. Ambrosi and M. Pumera, Chem. Eur. J. 22, 153 (2016). https://doi.org/10.1002/chem.201503110
A. L. Ivanovskii, Russ. Chem. Rev. 81, 571 (2012).
A. K. Geim and K. S. Novoselov, Nat. Mater. 6, 183 (2007).
F. Bonaccorso, L. Colombo, G. Yu, et al., Science (Washington, DC, U. S.) 347 (6217), 1246501 (2015). https://doi.org/10.1126/science.1246501
S. Shahgaldi and J. Hamelin, Carbon 94, 705 (2015). https://doi.org/10.1016/j.carbon.2015.07.055
J. Liu, H. J. Choi, and L.-Y. Meng, J. Ind. Eng. Chem. 64, 1 (2018). https://doi.org/10.1016/j.jiec.2018.02.021
H. Wang, T. Maiyalagan, and X. Wang, ACS Catal. 2, 781 (2012). https://doi.org/10.1021/cs200652y
J. Duan, S. Chen, M. Jaroniec, and S. Z. Qiao, ACS Catal. 5, 5207 (2015). https://doi.org/10.1021/acscatal.5b00991
Y. Chen, J. Wang, H. Liu, et al., J. Phys. Chem. C 115, 3769 (2011). https://doi.org/10.1021/jp108864y
K. Jukk, N. Kongi, P. Rauwel, et al., Electrocatalysis 7, 428 (2016). https://doi.org/10.1007/s12678-016-0322-1
Y. Deng, Y. Xie, K. Zou, and X. Ji, J. Mater. Chem. A 4, 1144 (2016). https://doi.org/10.1039/C5TA08620E
N. A. Kumar, H. Nolan, N. McEvoy, et al., J. Mater. Chem. A 1, 4431 (2013). https://doi.org/10.1039/c3ta10337d
A. Mueller, M. G. Schwab, N. Encinas, et al., Carbon 84, 426 (2015). https://doi.org/10.1016/j.carbon.2014.11.054
M. Rybin, A. Pereyaslavtsev, T. Vasilieva, et al., Carbon 96, 196 (2016). https://doi.org/10.1016/j.carbon.2015.09.056
N. Karthikeyan, B. P. Vinayan, M. Rajesh, et al., Fuel Cells 15, 278 (2015). https://doi.org/10.1002/fuce.201400134
O. K. Alekseeva, E. K. Lutikova, V. V. Markelov, et al., Int. J. Electrochem. Sci. 13, 797 (2018). https://doi.org/10.20964/2018.01.79
O. Alekseeva, A. Mikhalev, E. Lutikova, et al., Catalysts 8, 665 (2018). https://doi.org/10.3390/catal8120665
S. V. Akel’kina, A. S. Pushkarev, S. A. Grigoriev, I. V. Pushkareva, and V. N. Fateev, Russ. J. Electrochem. 54, 251 (2018).
S. A. Grigor’ev, A. S. Pushkarev, V. N. Kalinichenko, I. V. Pushkareva, M. Yu. Presnyakov, and V. N. Fateev, Kinet. Catal. 56, 689 (2015).
O. K. Alexeeva and V. N. Fateev, Int. J. Hydrogen Energy 41, 3373 (2016). https://doi.org/10.1016/j.ijhydene.2015.12.147
V. N. Fateev, O. K. Alekseeva, V. I. Porembskii, et al., Al’tern. Energet. Ekol., No. 25–27, 88 (2017). https://doi.org/10.15518/isjaee.2017.25-27.088-099
S. Grigoriev, V. Fateev, A. Pushkarev, et al., Materials 11, 1405 (2018). https://doi.org/10.3390/ma11081405
A. K. Mishra and S. Ramaprabhu, Desalination 282, 39 (2011). https://doi.org/10.1016/j.desal.2011.01.038
K. S. Kim, Y. Zhao, H. Jang, et al., Nature (London, U.K.) 457 (7230), 706 (2009). https://doi.org/10.1038/nature07719
Y. J. Oh, J. J. Yoo, Y. Kim Il, et al., Electrochim. Acta 116, 118 (2014). https://doi.org/10.1016/j.electacta.2013.11.040
J. Ma, A. Habrioux, Y. Luo, et al., J. Mater. Chem. A 3, 11891 (2015). https://doi.org/10.1039/C5TA01285F
A. Śliwak, B. Grzyb, N. Díez, and G. Gryglewicz, Appl. Surf. Sci. 399, 265 (2017). https://doi.org/10.1016/j.apsusc.2016.12.060
S. Ratso, I. Kruusenberg, M. Vikkisk, et al., Carbon 73, 361 (2014). https://doi.org/10.1016/j.carbon.2014.02.076
L. Stobinski, B. Lesiak, A. Malolepszy, et al., J. Electron Spectrosc. Relat. Phenom. 195, 145 (2014). https://doi.org/10.1016/j.elspec.2014.07.003
C. Botas, P. Álvarez, C. Blanco, et al., Carbon 52, 476 (2013). https://doi.org/10.1016/j.carbon.2012.09.059
D. Usachov, O. Vilkov, A. Grüneis, et al., Nano Lett. 11, 5401 (2011). https://doi.org/10.1021/nl2031037
P. Hu, K. Liu, C. P. Deming, and S. Chen, J. Chem. Technol. Biotechnol. 90, 2132 (2015). https://doi.org/10.1002/jctb.4797
Z. Wu, M. Song, J. Wang, and X. Liu, Catalysts 8, 196 (2018). https://doi.org/10.3390/catal8050196
L. Zhang and Z. Xia, J. Phys. Chem. C 115, 11170 (2011). https://doi.org/10.1021/jp201991
ACKNOWLEDGMENTS
We are grateful to E.V. Kukueva for obtaining images of the samples by TEM and SEM.
Funding
This study was funded by RFBR according to the research project no. 18-53-53025.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pushkarev, A.S., Alekseeva, O.K., Pushkareva, I.V. et al. Plasma Nitrogen Doping of Nanostructured Reduced Graphene Oxide. Nanotechnol Russia 15, 735–740 (2020). https://doi.org/10.1134/S1995078020060142
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995078020060142