Abstract
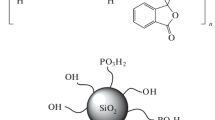
Sulfonated polyimides (SPIs)/silica composite membranes were prepared via an in situ sol–gel reaction followed by solution casting. SPIs based on 1,4,5,8-naphthalene tetracarboxylic dianhydride, 4,4'-diaminodiphenyl ether-2,2'-disulfonic acid and 4,4'-diaminodiphenyl ether have been chosen for the preparation of composite membranes due to their relatively high hydrolytic stability. Membranes varying in degree of sulfonation as well as silica content were characterized by FT-IR spectroscopy, thermal gravimetric analysis, transmission electron microscopy and impedance spectroscopy. It was found that the thermal and hydrolytic stability of membranes was enhanced by silica particles. Based on the morphological and conductivity data it is suggested that formation of a hydrogen bonded network of silanol groups of silica nanoparticles and sulfonic acid groups of the polymer matrix enhances the proton conductivity via the hopping mechanism of proton transport.




Similar content being viewed by others
REFERENCES
B. C. H. Steele and A. Heinzel, Nature (London, U.K.) 414, 345 (2001).
F. Barbir and S. Yazici, Int. J. Energy Res. 32, 369 (2008).
B. Smitha, S. Sridhar, and A. A. Khan, J. Membr. Sci. 259, 10 (2005).
K. A. Mauritz and R. B. Moore, Chem. Rev. 104, 4535 (2004).
M. A. Hickner, H. Ghassemi, Y. S. Kim, et al., Chem. Rev. 104, 4587 (2004).
C. Genies, R. Mercier, B. Sillion, et al., Polymer 42, 5097 (2001).
W. Jang, C. Lee, S. Sundar, et al., Polymer Degrad. Stab. 90, 431 (2005).
N. Asano, M. Aoki, S. Suzuki, et al., J. Am. Chem. Soc. 128, 1762 (2006).
Y. Yan, O. Yamada, K. Tanaka, and K. Okamoto, Polymer J. 38, 197 (2006).
J. Fang, X. Guo, S. Harada, et al., Macromolecules 35, 9022 (2002).
Y. Hongyan, K. Shi, N. Song, et al., Polymer 103, 171 (2016).
C. Lee, S. Sundar, J. Kwon, and H. Han, J. Polym. Sci., Part A 42, 3621 (2004).
J. Long, H. Yang, Y. Wang, et al., ChemElectroChem 7, 937 (2020).
N. Ali, F. Ali, S. Saeed, et al., J. Mater. Sci.: Mater. Electron. 30, 19164 (2019).
C. Genies, R. Mercier, B. Sillion, et al., Polymer 42, 359 (2001).
C. I. Filipoi, X. Zhu, D. Turp, et al., Int. J. Hydrogen. Energy 37, 14454 (2012).
S. T. Muntha, M. Ajmal, H. Naeem, et al., Polym. Compos. 40, 1897 (2019).
K. T. Adjemian, R. Dominey, L. Krishnan, et al., Chem. Mater. 18, 2238 (2006).
Z. G. Shao, P. Joghee, and I. M. Hsing, J. Membr. Sci. 229, 43 (2004).
S. T. Muntha, J. Ambreen, M. Siddiq, et al., JTCM (2019).
R. A. Zoppi and S. P. Nunes, J. Electroanal. Chem. 445, 39 (1998).
A. K. Sahu, G. Selvarani, S. Pitchumani, et al., J. Electrochem. Soc. 154, B123 (2007).
K. T. Adjemian, S. Srinivasan, J. Benziger, and A. B. Bocarsly, J. Power Sources 109, 356 (2002).
R. Jiang, H. R. Kunz, and M. J. Fenton, J. Membr. Sci. 272, 116 (2006).
I. Colicchio, D. E. Demco, M. Baias, et al., J. Membr. Sci. 337, 125 (2009).
Z. Gaowen and Z. Zhentao, J. Membr. Sci. 261, 107 (2005).
P. Musto, G. Ragosta, G. Scarinzi, and L. Mascia, Polymer 45, 1697 (2004).
S. Panero, P. Fiorenza, M. A. Navarra, et al., J. Electochem. Soc. 152, A2400 (2005).
C. H. Lee, S. Y. Hwang, J. Y. Sohna, et al., J. Power Sources 163, 339 (2006).
L. Zou, S. Roddecha, and M. Anthamatten, Polymer 50, 3136 (2009).
Funding
The authors greatly thank the Ministry of Science and Higher Education of the Russian Federation (contract no. 05.605.21.0188 from 3 December 2019 (RFMEFI60519X0188)) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gupta, M., Zhu, X., Melnikov, A.P. et al. Sulfonated Polyimide–Silica Composite Membranes: Preparation, Morphology and Proton Conductivity. Nanotechnol Russia 15, 778–784 (2020). https://doi.org/10.1134/S1995078020050043
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995078020050043