Abstract
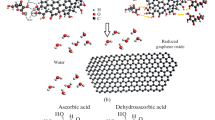
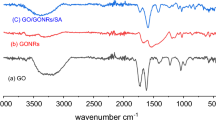
The preparation of hydro- and aerogels based on reduced graphite oxide and gold nanoparticles obtained by the method of Turkevich is described. According to the transmission electron microscopy data, gold nanoparticles have a spherical shape and an average size of 16‒18 nm. Sorption of methylene blue dye from water solutions was used to characterize the obtained 3D structures. The sorption properties of hydro- and aerogels were compared; it was shown that aerogels sorb the dye more efficiently (>80%) than a composite based on hydrogel (~50%). The formed porous 3D structure of the aerogel efficiently sorbs the molecules of the dye, while the gold nanoparticles facilitate its destruction under the visible light. Sorption by aerogels of pure graphite oxide is most efficient at room temperature, in a neutral medium, and in the absence of additional reducing agents. Introduction of gold nanoparticles to aerogels led to an increase of the maximal sorption by 15%.











Similar content being viewed by others
REFERENCES
A. K. Geim and K. S. Novoselov, “The rise of graphene,” Nat. Mater. 6, 183 (2007).
A. Lerf, H. He, M. Forster, and J. Klinowski, “Structure of graphite oxide revisited,” J. Phys. Chem. B 102, 4477 (1998).
Yanwu Zhu, Shanthi Murali, Weiwei Cai, et al., “Graphene and graphene oxide: synthesis, properties, and applications,” Adv. Mater. 22, 3906 (2010).
H. Gao and H. Duan, “2D and 3D graphene materials: preparation and bioelectrochemical applications,” Biosens. Bioelectron. 65, 404 (2015).
B. C. Brodie, “On the athomic weight of graphite,” Phil. Trans. R. Soc. London 149, 249 (1859).
W. S. Hammers and R. E. Offeman, “Preparation of graphitic oxide,” J. Am. Chem. Soc. 80, 1339 (1958).
D. C. Marcano, D. V. Kosynkin, J. M. Berlin, et al., “Improved synthesis of graphene oxide,” ACS Nano 4, 4806 (2010).
I. Kondratowicz, K. Zelechowska, M. Nadolska, et al., “Comprehensive study on graphene hydrogels and aerogels synthesis and their ability of gold nanoparticles adsorption,” Colloids Surf. A 528, 65 (2017).
W. Chen and L. Yan, “In situ self-assembly of mild chemical reduction graphene for three-dimensional architectures,” Nanoscale 3, 3132 (2011).
Xuan Lu, Xiaoli Liu, Ting Shen, et al., “Convenient fabrication of graphene/gold nanoparticle aerogel as direct electrode for H2O2 sensing,” Mater. Lett. 207, 49 (2017).
H. Gao and H. Duan, “2D and 3D graphene materials: Preparation and bioelectrochemical applications,” Biosens. Bioelectron., No. 65, 404 (2015).
Qiangqiang Zhang, Yu Wang, Baoqiang Zhang, et al., “3D superelastic graphene aerogel-nanosheet hybrid hierarchical nanostructures as high-performance supercapacitor electrodes,” Carbon, No. 127, 449 (2018).
Dong Ma, Jiantao Lin, Yuyun Chen, Wei Xue, Li-Ming Zhang, “In situ gelation and sustained release of an antitumor drug by graphene oxide nanosheets,” Carbon 50, 3001 (2012).
E. Jokara, S. Shahrokhiana, A. Iraji zada, et al., “An efficient two-step approach for improvement of graphene aerogel characteristics in preparation of supercapacitor electrodes,” J. Energy Storage, No. 17, 465 (2018).
Xin Wang, Chengxing Lu, Huifen Peng, et al., “Efficiently dense hierarchical graphene based aerogel electrode for supercapacitors,” J. Power Sources, No. 324, 188 (2016).
Zh. Sun, W. Fan, and T. Liu, “Graphene/graphene nanoribbon aerogels as tunable three-dimensional framework for efficient hydrogen evolution reaction,” Electrochim. Acta, No. 250, 91 (2017).
Zh. Yang, G. Xing, P. Hou, and D. Han, “Amino acid-mediated n-doped graphene aerogels and its electrochemical properties,” Mater. Sci. Eng. B, No. 228, 198 (2018).
Xuan Lu, Xiaoli Liu, Ting Shen, et al., “Convenient fabrication of graphene/gold nanoparticle aerogel as direct electrode for H2O2 sensing,” Mater. Lett., No. 207, 49 (2018).
R. Pocklanova, A. K. Rathi, M. B. Gawande, et al., “Gold nanoparticle-decorated graphene oxide: synthesis andapplication in oxidation reactions under benign conditions,” J. Mol. Catal. A: Chem. 424, 121 (2016).
Xiaoli Liu, Ting Shen, Zhiyong Zhao et al., “Graphene/gold nanoparticle aerogel electrode for electrochemical sensing of hydrogen peroxide,” Mater. Lett. 229, 368 (2018).
J. Xie, X. Yang, and X. Xu, “Wet chemical method for synthesizing 3D graphene/gold nanocomposite: catalytic reduction of methylene blue,” Phys. E (Amsterdam, Neth.) 88, 201 (2017).
Yu. V. Ioni, V. V. Voronov, A. V. Naumkin, E. Yu. Buslaeva, A. V. Egorov, S. V. Savilov, and S. P. Gubin, “Platinum, palladium, and rhodium nanoparticles on the surface of graphene flakes,” Russ. J. Inorg. Chem. 60, 709 (2015).
Yu. Ioni, E. Buslaeva, and S. Gubin, “Synthesis of graphene with noble metals nanoparticles on its surface,” Mater. Today: Proc. 3, 209 (2016).
Fang Ren, Zhen Li, Wen-Zhen Tan, et al., “Facile preparation of 3D regenerated cellulose/graphene oxide composite aerogel with high-efficiency adsorption towards methylene blue,” J. Colloid Interface Sci. 532, 58 (2018).
J. Turkevich, P. C. Stevenson, and J. Hillier, “A study of the nucleation and growth processes in the synthesis of colloidal gold,” J. Am. Chem. Soc., 55 (1951).
Funding
This study was supported by the Russian Foundation for Basic Research, project nos. 19-03-00556, 18-29-19120.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by V. Kudrinskaya
Rights and permissions
About this article
Cite this article
Eremina, E.A., Dobrovolskii, A., Lemesh, I.A. et al. 3D Structures Based on Reduced Graphite Oxide and Gold Nanoparticles and Their Sorption Properties. Nanotechnol Russia 14, 427–434 (2019). https://doi.org/10.1134/S1995078019050045
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995078019050045