Abstract
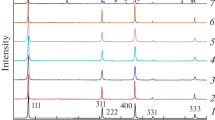
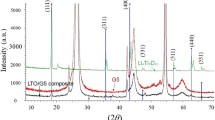
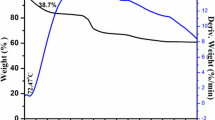
The influence of treatment temperature and a carbon precursor on the formation of a Li4Ti5O12-based anodic material and its electrochemical characteristics as part of a lithium-ion battery have been investigated. It is demonstrated that the variation of annealing temperature and the addition of saccharose prior to final annealing allow for the variation of Li4Ti5O12 particle sizes. At annealing temperatures of 400–600°C, lithium titanate forms as part of an anatase titanium oxide composite. Thermogravimetry, Raman spectroscopy, and electrochemical testing results show that a preannealing of the sample at temperatures of no less than 400°C and the addition of saccharose with subsequent annealing in an inert atmosphere are required for the formation of a conducting carbon coating. The formation of the carbon coating facilitates the inclusion of the anatase phase into the charging and discharging processes, which significantly increases the electrochemical capacity of samples obtained at low annealing temperature. The highest electrochemical capacity values (140 mA h/g) of anodic Li4Ti5O12 samples were obtained only after annealing at 800°C. We note the unexpected formation of carbon nanotubes in samples with a final annealing temperatures of 600°C.
Similar content being viewed by others
References
D. Yugović and D. Uskoković, J. Power Sources 190, 538 (2009).
J. B. Goodenough and K.-S. Park, J. Am. Chem. Soc. 135, 1167 (2013).
A. B. Yaroslavtsev, T. L. Kulova, and A. M. Skundin, Russ. Chem. Rev. 84, 826 (2015).
A. M. Skundin, O. N. Efimov, and O. V. Yarmolenko, Russ. Chem. Rev. 71, 329 (2002).
G. Kucinskis, G. Bajars, and J. Kleperis, J. Power Sources 240, 66 (2013).
M. V. Reddy, G. V. Subba Rao, and B. V. R. Chowdari, Chem. Rev. 113, 5364 (2013).
B. L. Ellis, K. Town, and L. F. Nazar, Electrochim. Acta 84, 145 (2012).
B. Xu, D. Qian, Z. Wang, and Y. S. Meng, Mater. Sci. Eng. R: Rep. 73, 51 (2012).
A. B. Yaroslavtsev, Russ. Chem. Rev. 78, 1013 (2009).
J. Maier, Z. Phys. Chem. 217, 415 (2003).
N. F. Uvarov and V. V. Boldyrev, Russ. Chem. Rev. 70, 265 (2001).
A. B. Yaroslavtsev, Nanotechnol. Russ. 7, 437 (2012).
P. P. Ferguson, A. D. W. Todd, and J. R. Dahn, Electrochem. Commun. 10, 25 (2008).
D. Ahn, X. Xiao, Y. Li, A. K. Sachdev, H. W. Park, A. Yu, and Zh. Chen, J. Power Sources 212, 66 (2012).
D. W. Murphy, R. J. Cava, S. M. Zahurak, and A. Santoro, Solid State Ionics 9–10, 413 (1983).
Z. G. Yang, D. Choi, S. Kerisit, K. M. Rosso, D. H. Wang, J. Zhang, G. Graff, and J. Liu, J. Power Sources 192, 588 (2009).
S. Schamer, W. Weppner, and P. Schmid-Beurmann, J. Electrochem. Soc. 146, 857 (1999).
D. V. Safronov, S. A. Novikova, T. L. Kulova, A. M. Skundin, and A. B. Yaroslavtsev, Inorg. Mater. 48, 57 (2012).
R. P. Vidano and D. B. Fishbach, Solid State Commun. 39, 341 (1961).
S. S. Bukalov, L. A. Makhalitsyn, Ya. V. Zubavichus, L. A. Leites, and Yu. N. Novikov, Ross. Khim. Zh. 1 (1), 83 (2006).
R. Baddour-Hadjean and J.-P. Pereira-Ramos, Chem. Rev. 110, 1278 (2010).
W. J. H. Borghols, M. Wagemaker, U. Lafont, E. M. Kelder, and F. M. Mulder, J. Am. Chem. Soc. 131, 17786 (2009).
U. Lafont, D. Carta, G. Mountjoy, A. V. Chadwick, and E. M. Kelder, J. Phys. Chem. C 114, 1372 (2010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © I.A. Stenina, S.S. Bukalov, T.L. Kulova, A.M. Skundin, N.Yu. Tabachkova, A.B. Yaroslavtsev, 2015, published in Rossiiskie Nanotekhnologii, 2015, Vol. 10, Nos. 11–12.
Rights and permissions
About this article
Cite this article
Stenina, I.A., Bukalov, S.S., Kulova, T.L. et al. Influence of a carbon coating on the electrochemical properties of lithium-titanate-based nanosized materials. Nanotechnol Russia 10, 865–871 (2015). https://doi.org/10.1134/S1995078015060130
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995078015060130