Abstract
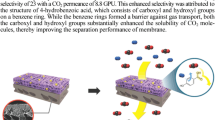
The work is devoted to exploring the possibility of using supercritical fluids as media for modification of polymers offering promise for production of gas-separation membranes with the goal to improve selectivity towards CO2. The possibility is demonstrated for introduction of fragments of quaternary ammonium salts into the structure of poly(4-methyl-2-pentyne) with the help of a two-stage process: bromination of the initial polymer with N-bromosuccinimide followed by the addition of the tertiary amine—N-butylimidazol— conducted in supercritical fluids as a medium. The use of trifluoromethane as the reaction medium provides the highest degree of modification of the brominated polymer with the amine. The polymer produced under the optimized conditions demonstrates a threefold increase of the calculated selectivity of separation of CO2 and N2 in comparison with the initial poly(4-methyl-2-pentyne).
Similar content being viewed by others

References
T. C. Merkel, H. Lin, X. Wei, and R. Baker, J. Membr. Sci. 359, 126 (2010).
A. Morisato and I. Pinnau, J. Membr. Sci. 121, 243 (1996).
B. C. Khotimsky, S. M. Matson, E. G. Litvinova, T. N. Bondarenko, and A. I. Rebrov, Polym. Sci., Ser. A 45, 740 (2003).
C. H. Lau, P. Li, F. Li, T.-S. Chung, and D. R. Paul, Prog. Polym. Sci. 38, 740 (2013).
J. E. Bara, C. J. Gabriel, E. S. Hatakeyama, S. Lessmann, R. D. Noble, and D. L. Gin, J. Membr. Sci. 321, 3 (2008).
R. S. Bhavsar, S. C. Kumbharkar, and U. K. Kharul, J. Membr. Sci. 470, 494 (2014).
H. L. Cong, B. Yu, J. G. Tang, and X. S. Zhao, J. Polym. Res. 19, 2 (2012).
T. Sakaguchi, H. Ito, T. Masuda, and T. Hashimoto, Polymer 54, 6709 (2013).
P. Li, Q. C. Zhao, J. L. Anderson, S. Varanasi, and M. R. Coleman, J. Polym. Sci., Part A: Polym. Chem. 48, 4036 (2010).
B. J. Adzima, S. R. Venna, S. S. Klara, H. K. He, M. J. Zhong, D. R. Luebke, M. S. Mauter, K. Matyjaszwski, and H. B. Nulwala, J. Mater. Chem. A 2, 7967 (2014).
V. G. Polevaya, G. N. Bondarenko, G. A. Shandryuk, V. D. Dolzhikova, and V. S. Khotimskiy, Russ. Chem. Bull. 4, 1067 (2016).
A. A. Masalev, V. S. Khotimskii, G. N. Bondarenko, and M. V. Chirkova, Polym. Sci., Ser. A 50, 37 (2008).
K. J. Stanger, J.-J. Lee, and B. D. Smith, J. Org. Chem. 72, 9663 (2007).
A. I. Cooper, J. Mater. Chem. 10, 207 (2000).
M. F. Kemmere and T. Meyer, Supercritical Carbon Dioxide: in Polymer Reaction Engineering (Wiley-VCH, Weinheim, 2005).
N. P. Volynskii, in Analysis Methods of Organic Compounds of Oil, their Mixtures and Derivatives, Collection of Articles (Nauka, Moscow, 1969), No. 2 [in Russian].
J. Qui, J.-M. Zheng, and K.-V. Peinemann, Macromolecules 39, 4093 (2006).
S. A. Legkov, G. N. Bondarenko, and Yu. V. Kostina, Polym. Sci., Ser. A 54, 187 (2012).
Y. Duan, P. Sun, S. Zhang, Z. Yao, X. Luo, and L. Ye, J. Fuel Chem. Technol. 43, 1113 (2015).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.G. Polevaya, A.M. Vorobei, O.I. Pokrovskiy, G.A. Shandryuk, O.O. Parenago, V.V. Lunin, V.S. Khotimskiy, 2017, published in Sverkhkriticheskie Flyuidy. Teoriya i Praktika, 2017, Vol. 12, No. 2, pp. 49–59.
Rights and permissions
About this article
Cite this article
Polevaya, V.G., Vorobei, A.M., Pokrovskiy, O.I. et al. Modification of Poly(4-Methyl-2-Pentyne) in Supercritical Fluid Medium for Production of CO2-Selective Gas-Separation Membranes. Russ. J. Phys. Chem. B 11, 1276–1282 (2017). https://doi.org/10.1134/S1990793117080085
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793117080085