Abstract
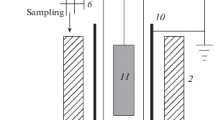
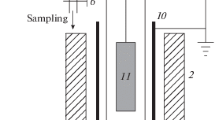
The possibility of controlling the rate of ethylene hydrogenation on a platinum nanocoating is established by applying to it electric potentials of different polarities and magnitudes from an external voltage source. At a negative potential of −10 V, the hydrogenation rate increases by 4%, whereas at a positive potential of +10 V, the hydrogenation rate increases by 42% under the conditions of the experiment at room temperature, atmospheric pressure, and an initial mixture composition of 0.09C2H4 + 0.91H2. Quantum-chemical calculations of the energy of the reaction of platinum hydride with hydrogen, Pt2H2 + H2 → Pt2H3 + H, and the energy characteristics of similar reactions involving negatively and positively charged Pt2H2 are performed. It has been demonstrated that the presence of a negative or positive charge on Pt2H2 lowers the endothermicity of formation of H radicals by 18.4 or 22.5 kcal/mol, respectively. Based on the calculation results, a mechanism is proposed to explain the effect of the charge of a platinum coating on its catalytic activity in ethylene hydrogenation.
Similar content being viewed by others
References
V. I. Bukhtiyarov and M. G. Slin’ko, Russ. Chem. Rev. 70, 147 (2001).
B. Roldan Cuenya, Thin Solid Films 518, 3127 (2010).
F. Behafarid and B. Roldan Cuenya, Top. Catal. 56, 1542 (2013).
N. T. Fofang, T. S. Luk, M. Okandan, G. N. Nielson, and I. Brener, Opt. Express 21, 4774 (2013).
E. Martinsson, M. A. Otte, M. M. Shahjamali, B. Sepulveda, and D. Aili, J. Phys. Chem. 118, 24680 (2014).
E. Hazrati, G. Brocks, and G. A. de Wijs, J. Phys. Chem. 118, 5102 (2014).
M. V. Grishin, A. K. Gatin, N. V. Dokhlikova, et al., Kinet. Catal. 56, 532 (2015).
M. V. Grishin, A. K. Gatin, V. G. Slutskii, V. A. Kharitonov, S. A. Tsyganov, and B. R. Shub, Russ. J. Phys. Chem. B 10, 538 (2016).
M. V. Grishin, A. K. Gatin, V. G. Slutskii, V. A. Kharitonov, B. R. Shub, and S. A. Tsyganov, Russ. J. Phys. Chem. B 7, 383 (2013).
M. V. Grishin, A. K. Gatin, V. G. Slutskii, V. A. Kharitonov, and B. R. Shub, Russ. J. Phys. Chem. B 9, 596 (2015).
M. V. Grishin, A. K. Gatin, V. G. Slutskii, V. A. Kharitonov, S. A. Tsyganov, and B. R. Shub, Russ. J. Phys. Chem. B 35, 760 (2016).
T. Jacob and W. A. Goddart, J. Phys. Chem. B 108, 8311 (2004).
J. Greeley and M. Mavrikakis, J. Phys. Chem. B 109, 3460 (2005).
O. Yasuharu, Chem. Phys. Lett. 429, 209 (2006).
Y. Ishikawa, J. J. Mateo, D. A. Tryk, and C. R. Cabrera, J. Electroanal. Chem. 607, 37 (2007).
A. S. Zyubin, T. S. Zyubina, Yu. A. Dobrovol’skii, V. M. Volokhov, and Z. G. Bazhanova, Russ. J. Inorg. Chem. 56, 1290 (2011).
A. S. Zyubin, T. S. Zyubina, Yu. A. Dobrovol’skii, and V. M. Volokhov, Russ. J. Inorg. Chem. 57, 1460 (2012).
T. Ozaki, Phys. Rev. B 67, 155108 (2003).
T. Ozaki and H. Kino, Phys. Rev. B 69, 195113 (2004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.N. Korchak, M.V. Grishin, M.Ya. Bykhovskii, A.K. Gatin, V.G. Slutskii, V.A. Kharitonov, S.A. Tsyganov, B.R. Shub, 2017, published in Khimicheskaya Fizika, 2017, Vol. 36, No. 11, pp. 29–33.
Rights and permissions
About this article
Cite this article
Korchak, V.N., Grishin, M.V., Bykhovskii, M.Y. et al. Ethylene Hydrogenation on a Platinum Nanocoating at Various Electric Potentials. Russ. J. Phys. Chem. B 11, 932–936 (2017). https://doi.org/10.1134/S1990793117060057
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793117060057