Abstract
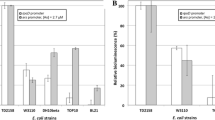
A procedure involving specific lux biosensors has been applied to rapid detection of environmental ammonium perchlorate (AP) under both laboratory and field conditions. The procedure is based on evaluating the influence of AP on the level of bioluminescence of lux biosensors. Two lux biosensors—Escherichia coli MG1655 katG::kan (pKatG-lux) and E. coli MG1655 (pSoxS-lux)—have been employed in testing the ability of AP to cause an oxidative stress associated with the appearance of hydrogen peroxide and superoxide anion radicals in the cell. The lux biosensors designed contain hybrid plasmids with appropriate regulatory DNA regions transcriptionally fused with the bacterial luciferase lux genes as reporter genes. AP at concentrations of 5–50 mmol/L exerts an effect on the induction of luminescence of the lux biosensors with the PkatG and PsoxS promoters. The intensity of the bioluminescence of the cells that contain the pKatG-lux and pSoxS-lux plasmids and have been treated with AP is 5–10 higher than the intensity of the bioluminescence of the untreated cells. Therefore, AP induces an oxidative stress in the bacterial cells through the formation of reactive oxygen species, namely, hydrogen peroxide and the superoxide anion radical. The high sensitivity and specificity of the lux biosensors makes them usable in AP detection in the environment.
Similar content being viewed by others
References
C. A. Vollmer and T. K. van Dyk, Adv. Microb. Physiol. 49, 131 (2004).
L. Galluzzi and M. Karp, Comb. Chem. High Thorough-put Screen 9, 501 (2006).
M. Woutersen, S. Belkin, B. Brouwer, et al., Anal. Bioanal. Chem. 400, 914 (2011).
V. Yu. Kotova, I. V. Manukhov, and G. B. Zavilgelskii, Appl. Biochem. Microbiol. 46, 781 (2010).
G. B. Zavil’gel’skii, V. Yu. Kotova, and A. S. Mironov, Russ. J. Phys. Chem. B 9, 454 (2015).
T. K. van Dyk and R. A. Rosson, Methods in Molecular Biology, Vol. 102: Bioluminescence Methods and Protocols, Ed. by R. A. Rosson (Humana, Totowa, NJ, 1998), p. 85.
J. Sambrook and D. W. Russell, Molecular Cloning: A Laboratory Manual, 3rd ed. (Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, 2001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.P. Balabanov, S.A. Khrulnova, V.Yu. Kotova, G.B. Zavilgelsky, 2017, published in Khimicheskaya Fizika, 2017, Vol. 36, No. 8, pp. 45–47.
Rights and permissions
About this article
Cite this article
Balabanov, V.P., Khrulnova, S.A., Kotova, V.Y. et al. Ammonium perchlorate detection in natural environments using specific lux biosensors. Russ. J. Phys. Chem. B 11, 663–665 (2017). https://doi.org/10.1134/S1990793117040133
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793117040133