Abstract
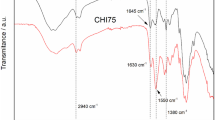
A new organogelator, L-phenylalanine dihydrazide derivative (BOC-Phe-HdHz) has been designed, synthesized and characterized. In addition, the gelling behaviors of BOC-Phe-HdHz were studied. The organogelator has been shown to be capable of forming stable thermoreversible organogels in various organic solvents at extremely low concentrations (<3 wt %). The gel–sol phase transition temperatures (T GS) were determined as a function of gelator concentration and the corresponding enthalpies (ΔH g) were extracted. SEM revealed that the gelator self-assembled into different supramolecular network structures in different gels. FT-IR confirmed that hydrogen bonding and hydrophobic interaction were the driving forces for the supramolecular assembly process. XRD was measured in three different states of the gelator: solid powder, gel and xerogel. In addition, XRD and molecular modeling studies have been carried out and provided more information of the possible packing modes for the formation of organogelator aggregates.
Similar content being viewed by others
References
R. G. Weiss, P. Terech, Molecular Gels: Materials with Self-Assembled Fibrillar Networks (Springer, Dordrecht, Netherlands, 2006)
M. D. Segarra-Maset, V. J. Nebot, J. F. Miravet, et al., Chem. Soc. Rev. 42, 7086 (2013).
X. D. Yu, X. H. Cao, L. M. Chen, et al., Soft Matter 8, 3329 (2012).
C. Wang, D. Q. Zhang, J. F. Xiang, et al., Langmuir 23, 9195 (2007).
K. Lalitha, P. Jenifer, Y. S. Prasad, et al., RSC Adv. 4, 48433 (2014).
I. V. Kumpanenko, A. V. Roschin, V. V. Usin, N. A. Ivanova, A. V. Bloshenko, A. E. Goncharova, and N. A. Sakharova, Russ. J. Phys. Chem. B 8, 720 (2014).
S. Bhattacharya and Y. Krishnan-Ghosh, Chem. Commun. 2, 185 (2001).
J. H. Jung, Y. Ono, K. Hanabusa, et al., J. Am. Chem. Soc. 122, 5008 (2000).
L. N. Lucas, J. V. Esch, R. M. Kellogg, et al., Chem. Commun., 759 (2001).
S. Mukherjee, G. R. Krishna, B. Mukhopadhyay, et al., Cryst. Eng. Comm. 17, 3345 (2015).
Y. Jiang, F. Zeng, R. Gong, et al., Soft Matter 9, 7538 (2013).
A. Hemamalini and T. M. Das, RSC Adv. 4, 41010 (2014).
S. Maji, A. Das, P. K. Sarkar, et al., RSC Adv. 4, 44650 (2014).
M. M. Piepenbrock, G. O. Lloyd, N. Clarke, et al., Chem. Rev. 110, 1960 (2010).
D. V. Zlenko and S. V. Stovbun, Russ. J. Phys. Chem. B 8, 499 (2014).
S. V. Stovbun, A. M. Zanin, A. A. Skoblin, A. I. Mikhailov, R. G. Kostyanovskii, M. V. Grishin, and B. R. Shub, Russ. J. Phys. Chem. B 5, 1019 (2011).
M. George and R. G. Weiss, Acc. Chem. Res. 39, 489 (2006).
J. V. Esch, Langmuir 25, 8392 (2009).
P. F. Duan and M. H. Liu, Langmuir 25, 8706 (2009).
K. G. Ragunathan and S. Bhattacharya, Chem. Phys. Lipids 77, 13 (1995).
K. Hanabusa, R. Tanaka, M. Suzuki, et al., Adv. Mater. 9, 1095 (1997).
D. Khatua and J. Dey, Langmuir 21, 109 (2005).
M. Suzuki, T. Sato, H. Shirai, and K. Hanabusa, New J. Chem. 30, 1184 (2006).
J. G. Hardy, A. R. Hirst, I. Ashworth, et al., Tetrahedron 63, 7397 (2007).
A. Motulsky, M. Lafleur, A. C. Couffin-Hoarau, et al., Biomaterials 26, 6242 (2005).
J. V. Esch, F. Schoonbeek, M. de Loos, et al., Chem.-Eur. J. 5, 937 (1999).
P. Terech, C. Rossat, and F. Volino, Colloid Interf. Sci. 227, 363 (2000).
A. Carré, P. le Grel, and M. Baudy-Floc’h, Tetrahedron Lett. 42, 1887 (2001).
S. H. Seo and J. Y. Chang, Chem. Mater. 17, 3249 (2005).
A. R. Hirst, D. K. Smith, M. C. Feiters, et al., Langmuir 20, 7070 (2004).
S. K. Samanta, A. Pal, and S. Bhattacharya, Langmuir 25, 8567 (2009).
H. Basit, A. Pal, S. Sen, et al., Chem.-Eur. J. 14, 6534 (2008).
T. Kar, S. Debnath, D. Das, et al., Langmuir 25, 8639 (2009).
G. Palui, A. Banerjee, Phys. Chem. B 112, 10107 (2008).
W. D. Jang, D. L. Jiang, and T. Aida, J. Am. Chem. Soc. 122, 3232 (2000).
W. D. Jang and T. Aida, Macromolecules 36, 8461 (2003).
M. Suzuki, T. Sato, A. Kurose, et al., Tetrahedron Lett. 4, 2741 (2005).
C. H. Tan, L. H. Su, R. Lu, et al., Mol. Liq. 124, 32 (2006).
Author information
Authors and Affiliations
Corresponding authors
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Yu, Y., Chu, N., Li, X.Y. et al. Synthesis, characterization and gelation mechanism of L-phenylalanine-based dihydrazide derivative as excellent gelator. Russ. J. Phys. Chem. B 11, 121–128 (2017). https://doi.org/10.1134/S1990793117010134
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793117010134