Abstract
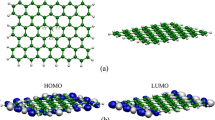
The adsorption properties of two types of naphthenic acids (NAs), benzoic acid and cyclohexane carboxylic acid on four-nitrogen coordinated transition-metal (Mn, Fe, Co, Ni, Cu, and Zn) embedded graphene (TMN4-G) were investigated in detail by means of density functional theory method. The calculation results indicate that NAs prefer the perpendicular adsorption configuration by bonding interactions between their carbonyl oxygen atom and TMN4 active site, and could be chemisorbed on FeN4-G, MnN4-G, and ZnN4-G. The FeN4-G gives the strongest adsorption to the NAs, indicating it is the best adsorbent among them. Electron density maps further confirm that NAs are chemically adsorbed on the FeN4-G surface, accompanied by electron transfer in the adsorption systems. The calculations indicate that benzoic acid has relatively stronger adsorption energy than that of cyclohexane carboxylic acid for the perpendicular adsorption on TMN4-G surface.
Similar content being viewed by others
References
J. S. Clemente and P. M. Fedorak, Chemosphere 60, 585 (2005).
F. M. Holowenko, M. D. MacKinnon, and P. M. Fedorak, Water. Res. 36, 2843 (2002).
S. J. Rowland, C. E. West, D. Jones, A. G. Scarlett, R. A. Frank, and L. M. Hewitt, Environ. Sci. Technol. 45, 9806 (2011).
B. Wang, Y. Wan, Y. Gao, G. Zheng, M. Yang, S. Wu, and J. Hu, Environ. Sci. Technol. 49, 5796 (2015).
E. Slavcheva, B. Shone, and A. Turnbull, Brit. Corros. J. 34, 125 (1999).
R. A. Frank, R. Kavanagh, B. K. Burnison, G. Arsenault, J. V. Headley, K. M. Peru, G. van der Kraak, and K. R. Solomon, Chemosphere 72, 1309 (2008).
S. S. Leung, M. D. MacKinnon, anbd R. E. Smith, Aquat. Toxicol. 62, 11 (2003).
K. E. Tollefsen, K. Petersen, and S. J. Rowland, Environ. Sci. Technol. 46, 5143 (2012).
V. V. Rogers, M. Wickstrom, K. Liber, and M. D. MacKinnon, Toxicol. Sci. 66, 347 (2002).
K. Thomas, K. Langford, K. Petersen, A. J. Smith, and K. E. Tollefsen, Environ. Sci. Technol. 43, 8066 (2009).
D. C. L. Wong, R. van Compernolle, J. G. Nowlin, D. L. O’Neal, and G. M. Johnson, Chemosphere 32, 1669 (1996).
L. Zou, B. Han, H. Yan, K. L. Kasperski, Y. Xu, and L. G. Hepler, J. Colloid. Interf. Sci. 190, 472 (1997).
K. A. R. Gomari, R. Denoyel, and A. A. Hamouda, J. Colloid. Interf. Sci. 297, 470 (2006).
R. A. Frank, R. Kavanagh, B. K. Burnison, J. V. Headley, K. M. Peru, G. van der Kraak, and K. R. Solomon, Chemosphere 64, 1346 (2006).
M. H. Mohamed, L. D. Wilson, J. V. Headley, and K.M. Peru, Process. Safety Environ. Protect. 86, 237 (2008).
A. K. Geim, Science 324, 1530 (2009).
J. K. Wassei and R. B. Kaner, Acc. Chem. Res. 46, 2244 (2013).
O. Leenaerts, B. Partoens, and F. Peeters, Phys. Rev. B 77, 125416 (2008).
M. Oubal, S. Picaud, M. T. Rayez, and J. C. Rayez, Comput. Theor. Chem. 1016, 22 (2013).
K. Doi, I. Onishi, and S. Kawano, Comput. Theor. Chem. 994, 54 (2012).
F. Ma, Z. Zhang, H. Jia, X. Liu, Y. Hao, and B. Xu, J. Mol. Struct.: THEOCHEM 955, 134 (2010).
N. Ding, X. Lu, and C. M. L. Wu, Comput. Mater. Sci. 51, 141 (2012).
D. Wang, Z. Yang, L. C. Xu, X. Liu, R. Liu, and X. Li, Comput. Theor. Chem. 1062, 84 (2015).
N. F. Domancich, R. M. Ferullo, and N. J. Castellani, Comput. Theor. Chem. 1059, 27 (2015).
Y. Mao, J. Yuan, and J. Zhong, J. Phys.: Condens. Matter 20, 115209 (2008).
C. Rajesh, C. Majumder, H. Mizuseki, and Y. Kawazoe, J. Chem. Phys. 130, 124911 (2009).
G. Zhao, L. Jiang, Y. He, J. Li, H. Dong, X. Wang, and W. Hu, Adv. Mater. 23, 3959 (2011).
O. G. Apul, Q. Wang, Y. Zhou, and T. Karanfil, Water. Res. 47, 1648 (2013).
Z. Pei, L. Li, L. Sun, S. Zhang, X. Q. Shan, S. Yang, and B. Wen, Carbon 51, 156 (2013).
F. F. Liu, J. Zhao, S. Wang, P. Du, and B. Xing, Environ. Sci. Technol. 48, 13197 (2014).
M. Lefèvre, E. Proietti, F. Jaouen, and J. P. Dodelet, Science 324, 71 (2009).
U. I. Kramm, J. Herranz, N. Larouche, T. M. Arruda, M. Lefevre, F. Jaouen, P. Bogdanoff, S. Fiechter, I. Abs-Wurmbach, S. Mukerjee, and J. P. Dodelet, Phys. Chem. Chem. Phys. 14, 11673 (2012).
B. Delley, J. Chem. Phys. 113, 7756 (2000).
B. Delley, Comput. Mater. Sci. 17, 122 (2000).
C. Lee, W. Yang, and R. G. Parr, Phys. Rev. B 37, 785 (1988).
S. A. Umoren, I. B. Obot, E. E. Ebenso, P. C. Okafor, O. Ogbobe, and E. E. Oguzie, Anti-Corros. Methods Mater. 53, 277 (2006).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Ma, L., Chen, X. Adsorption of naphthenic acids to the nitrogen-coordinated transition-metal embedded graphene: A DFT study. Russ. J. Phys. Chem. B 10, 1027–1031 (2016). https://doi.org/10.1134/S1990793116060233
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793116060233