Abstract
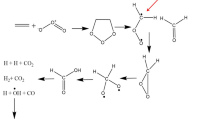
The results of quantum-chemical calculations of the kinetics and mechanism of the gas-phase thermal reactions of synthesis and decomposition of carbon suboxide are presented. The role of carbenes in the individual elementary stages is considered. The energy parameters of these stages and structures of intermediates formed in them are determined. The calculations are performed using the Møller-Plesset MP2 perturbation theory and the Dunning aug-cc-pVDZ correlated basis set with allowance for the zero-point-vibration energy (ZPE). The possibility of the formation of oxygen-containing C4O and C5O carbenes, which are formed in the dissociation of vibrationally excited chain oxides, produced, in turn, in the recombination of the carbon suboxide decomposition products, is for the first time predicted.
Similar content being viewed by others
References
L. B. Dashkevich and V. G. Beilin, Russ. Chem. Rev. 36, 391 (1967).
Yu. A. Kolbanovskii, Yu. A. Borisov, and A. M. Tsedilin, Dokl. Phys. Chem. 58, 180 (2013).
A. Klemenc, R. Wechsberg, and G. Wagner, Mh. Chem. 66, 337 (1935).
H. Palmer and T. J. Hirt, J. Am. Chem. Soc. 84, 113 (1962).
H. G. Wagner, P. A. Vlasov, K. J. Dorge, et al., Kinet. Catal. 42, 583 (2001).
H. Okabe, Photochemistry of Small Molecules (Wiley, New York, 1978).
D. J. Anderson and R. N. Rosenfeld, J. Chem. Phys. 94, 7857 (1991).
C. J. Bennett, C. S. Jamieson, and R. I. Kaiser, Planet. Space Sci. 56, 1181 (2008).
A. V. Emelianov, A. V. Eremin, A. A. Makeich, and V. E. Fortov, JETP Lett. 87, 470 (2008).
J. McFarlane, J. C. Polanyi, J. G. Shapter, and J. M. Williamson, J. Photochem. Photobiol. A 46, 139 (1989).
K. S. Pitzer and E. Clementi, J. Am. Chem. Soc. 81, 4477 (1959).
L. V. Gurvich, G. V. Karachentsev, and V. N. Kondrat’ev, Energy of Chemical Bond Cleavage. Ionization Potentials and Affinity to the Electron (Nauka, Moscow, 1974) [in Russian].
C. Moller and S. Plesset, Phys. Rev. 46, 618 (1934).
D. E. Woon and T. H. Dunning, Jr., J. Chem. Phys. 98, 1358 (1993).
C. Gonzalez and H. B. Schlegel, J. Phys. Chem. 94, 5523 (1990).
H. B. Schlegel and M. A. Robb, Chem. Phys. Lett. 93, 43 (1982).
E. Tschuikow-Roux and S. Kodama, J. Chem. Phys. 50, 5297 (1969).
V. O. Kompanets, V. B. Laptev, A. A. Makarov, et al., Russ. J. Phys. Chem. A 87, 794 (2013).
H. Reisler, M. Mangir, and C. Wittig, J. Chem. Phys. 73, 2280 (1980).
Yu. A. Kolbanovskii and Yu. A. Borisov, Mendeleev Commun. 21, 305 (2011).
A. V. Eremin, Energy Combust. Sci. 38, 1 (2012).
E. Miyoshi and N. Shida, Chem. Phys. Lett. 303, 50 (1999).
J. Koput, Chem. Phys. Lett. 320, 237 (2000).
M. Tonimoto, K. Kuchitsu, and Y. Moreno, Bull. Chem. Soc. Jpn. 43, 2776 (1970).
P. Bunker, J. Mol. Spectrosc. 80, 422 (1980).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © Yu.A. Kolbanovskii, A.M. Tsedilin, Yu.A. Borisov, 2014, published in Khimicheskaya Fizika, 2014, Vol. 33, No. 11, pp. 55–66.
Rights and permissions
About this article
Cite this article
Kolbanovskii, Y.A., Tsedilin, A.M. & Borisov, Y.A. A theoretical study of the role of carbenes in the kinetics and mechanism of the reactions of synthesis and pyrolysis of carbon suboxide. Russ. J. Phys. Chem. B 8, 829–840 (2014). https://doi.org/10.1134/S1990793114110050
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793114110050