Abstract
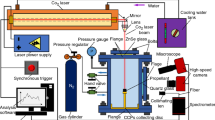
The combustion behavior of nano-aluminum-water (n-Al-H2O) mixture with addition of polyacrylamide (PAM) was investigated in argon at 0.1∼1.5 MPa using a constant-pressure strand burner. The burning rates of n-Al-H2O mixture were measured. The results show that PAM addition can not only help improve the burning rate of n-Al-H2O mixture, but also decrease the pressure index of burning rate. The mixture of n-Al powder and H2O cannot be ignited in argon at 0.1 MPa, but the mixture of n-Al powder and H2O with the 3 wt % PAM can be ignited, and the mixture can support the self-sustaining combustion. The burning rate is 7.64 mm/s. Moreover, the burning rate increases with increasing the pressure. In addition, the combustion process and flame image characteristics were obtained by a high-speed photography technique, and the element composition and surface morphology of the condensed combustion products were evaluated using a scanning electron microscopy combined with energy dispersive X-ray system.
Similar content being viewed by others
References
A. Ingenito and B. Claudio, J. Propuls. Power 20, 1056 (2004).
Y. L. Sun and B. Z. Zhu, Ind. Eng. Chem. Res. 50, 14136 (2011).
E. Shafirovich, V. Diakov, and A. Varma, Combust. Flame 144, 415 (2006).
R. J. Gill, C. Badiola, and E. L. Dreizin, Combust. Flame 157, 2015 (2010).
C. Badiola, R. J. Gill, and E. L. Dreizin, Combust. Flame 158, 2064 (2011).
T. Bazyn, H. Krier, and N. Glumac, Proc. Combust. Inst. 31, 2021 (2007).
S. Gallier, F. Sibe, and O. Orlandi, Proc. Combust. Inst. 33, 1949 (2011).
T. Bazyn, H. Krier, and N. Glumac, Combust. Flame 145, 703 (2006).
F. Franzoni, M. Milani, L. Montorsi, and V. Golovitchev, Int. J. Hydrogen Energy 35, 1548 (2010).
S. Álvarez-Barcia and J. R. Flores, Chem. Phys. 374, 131 (2010).
T. F. Miller and J. D. Herr, AIAA J., 2004-4037 (2004).
A. Sharipov, N. Titova, and A. Starik, J. Phys. Chem. A 115, 4476 (2011).
Y. L. Sun, Y. Tian, and S. F. Li, Chin. J. Chem. Phys 21, 245 (2008).
G. A. Risha, J. L. Sabourin, V. Yang, et al., Combust. Sci. Technol. 180, 2127 (2008).
G. A. Risha, S. F. Son, R. A. Yetter, et al., Proc. Combust. Inst. 31, 2029 (2007).
J. L. Sabourin, G. A. Risha, R. A. Yetter, et al., Combust. Flame 154, 587 (2008).
V. G. Ivanov, O. V. Gavrilyuk, O. V. Glaskov, et al., Combust. Explos. Shock Waves 36, 213 (2000).
Y. L. Sun, B. Z. Zhu, H. C. Dang, et al., J. Mater. Sci. 46, 4471 (2011).
V. A. Babuk, I. N. Dolotkazin, and A. A. Glebov, Propell. Explos. Pyrotech. 30, 281 (2005).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Zhu, B., Sun, Y. & Sun, H. Enhanced Al-H2O-based fuels combustion characteristics with polyacrylamide at low pressures. Russ. J. Phys. Chem. B 8, 492–498 (2014). https://doi.org/10.1134/S1990793114040289
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793114040289