Abstract—
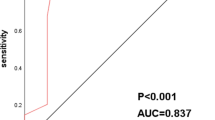
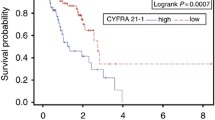
Non-small cell lung cancer (NSCLC) accounts for most cases of lung cancer. Histologically, it is subdivided into two histological subtypes: adenocarcinoma (AC) and squamous cell carcinoma (SCC). The five-year survival rate of patients with stage I NSCLC is two times higher than in patients with stage II and more than five times higher than in stage III−IV lung cancer patients. Currently, there are no informative blood biomarkers to diagnose early stages of NSCLC. The aim of this study was to evaluate complex determination of hyaluronic acid (HA), lymphocyte CXCR2 and granulocyte CXCR1 levels in the blood of patients with AC and SCC. Blood samples from 107 patients with SCC, 90 patients with AC, and 40 healthy people were used in this study. Concentration of HA in blood serum was determined by enzyme linked immunoassay. The level of CXCR2 and CXCR1 was determined by flow cytometry. Diagnostic parameters were determined by constructing mathematical models in the form of regression equations using the method of stepwise inclusion of predictors and subsequent ROC-analysis. Results of the study indicate that MFI CXCR1 in granulocytes, the proportion of lymphocytes expressing CXCR2 and concentration of HA in blood serum in stage I AC and SCC are significantly higher than in healthy people. The level of these indicators significantly increases at stage II of the disease compared to stage I and demonstrates further growth at its later stages. Based on obtained results, regression equations were created. These included: (i) MFI CXCR1 in granulocytes, the proportion of lymphocytes supplied with CXCR2 and the HA serum concentration in to detect stages I−II SCC (diagnostic sensitivity 95.7%, specificity 93.7%, threshold value 0.59) and stages III−IV SCC (diagnostic sensitivity 93.1%, specificity 93.3%, threshold value 0.64); (ii) the proportion of lymphocytes supplied with CXCR2, MFI CXCR1 in granulocytes and CYFRA 21-1 blood level, for detection of I−II stages of AC (sensitivity 91.3%, specificity 94.7%, threshold value 0.61); (iii) the proportion of lymphocytes expressing CXCR2 and CYFRA 21-1 blood level, for detection of AC stages III–IV (sensitivity 94.6%, specificity 91.3%, threshold value 0.15); (IV) the proportion of lymphocytes expressing CXCR2 and serum HA level to differentiate stage II SCC from stage I (sensitivity 94.4%, specificity 87.5%, threshold value 0.44) and II stage AC from stage I (sensitivity 88.5%, specificity 91.2%, threshold value 0.46).

Similar content being viewed by others
REFERENCES
Sung, H., Ferlay, J., Siegel, R.L., Laversanne, M., and Soerjomataram, I., CA Cancer J. Clin., 2020, vol. 71, no. 3, pp. 209–249. https://doi.org/10.3322/caac.21660
Raso, M.G. and Wistuba, I.I., J. Thoracic Oncol., 2007, vol. 2, no. 7, pp. 128–135. https://doi.org/10.1097/JTO.0b013e318074fe42
Rosen, R.D. and Sapra, A., TNM Classification, StatPearls Publishing, NCBI Bookshelf, 2021.
Wang, B.Y., Huang, J.Y., Chen, H.C., Lin, C.H., Lin, S.H., Hung, W.H., and Cheng, Y.F., J Cancer Res. Clin. Oncol., 2020, vol. 146, no. 1, pp. 43–52. https://doi.org/10.1007/s00432-019-03079-8
Kulpa, J., Wójcik, E., Reinfuss, M., and Kołodziejski, L., Clin. Chem., 2002, vol. 48, no. 11, pp. 1931–1937.
Liu, Q., Li, A., Tian, Y., Wu, J.D., Liu, Y., Li, T., Chen, Y., Han, X., and Wu, K., Cytokine Growth Factor Rev., 2016, vol. 31, pp. 61–71. https://doi.org/10.1016/j.cytogfr.2016.08.002
Wang, L., Shi, L., Gu, J., Zhan, C., Xi, J., Ding, J., and Ge, D., J. Physiol. Biochem., 2018, vol. 74, no. 2, pp. 313–324. https://doi.org/10.1007/s13105-018-0619-z
Pirinen, R., Tammi, R., Tammi, M., Hirvikoski, P., Parkkinen, J.J., Johansson, R., Böhm, J., Hollmén, S., and Kosma, V.M., Int. J. Cancer, 2001, vol. 95, no. 1, pp. 12–17. https://doi.org/10.1002/1097-0215(20010120)95:1<12:: aid-ijc1002>3.0.co;2-e
Bamji-Stocke, S., van Berkel, V., and Frieboes, B.H., Metabolomics, 2018, vol. 14, no. 6, 81. https://doi.org/10.1007/s11306-018-1376-2
Zhenyu, S., Chen, X., and Wang, X., Biomarker Res., 2016, vol. 4, 11. https://doi.org/10.1186/s40364-016-0065-4
Liu, Y., Wu, B., Geng, H., Xu, M., and Zhong, H., Oncol. Lett., 2015, vol. 10, no. 3, pp. 1315–1322. https://doi.org/10.3892/ol.2015.3402
Rangel, M.P., de Sá, V.K., Martins, V., Mar-tins, J.R.M., Parra, E.R., Mendes, A., Andrade, P.S., Reis, R.M., Longatto-Filho, A., Oliveira, C.Z., Takagaki, T., Carraro, D.M., Nader, H.B., and Capelozzi, V.L., Braz. J. Med. Biol. Res., 2015, vol. 48, no. 6, pp. 557–567. https://doi.org/10.1590/1414-431X20144300
Lin, W., Yugang, L., Yuefeng, M., Chao, D., Huijun, Z., Zhenghui, L., Zhenning, G., and Changsheng, Z., Cell Cycle, 2019, vol. 18, no. 24, pp. 3456–3471. https://doi.org/10.1080/15384101.2019.1689471
Rajul, P., Mathai, A., Shefali, S., Chandra, S., and Ravi, T., Indian J. Ophtalmol., 2008, vol. 56, no. 1, pp. 45–50. https://doi.org/10.4103./0301-4738.37595
Fang, R., Zhu, Y., Khadka1,V.S., Zhang, F., Jiang, B., and Deng, Y., Front. Physiol., 2018, vol. 9, 1710. https://doi.org/10.3389/fphys.2018.01710
Li, X., Asmitananda, T., Gao, L., Gai, D., Song, Z., Zhang, Y., Ren, H., Yang, T., Chen, T., and Chen, M., Neoplasma, 2012, vol. 59, no. 5, pp. 500–507.
Hernandez-Hernandez, J.R., Garcia-Garcia, J.M., and Martinez, M.T., Int. J. Biol. Markers, 1995, vol. 10, no. 3, pp. 149–155. https://doi.org/10.1177/172460089501000304
Funding
This work was financially supported by the budget of the Republic of Belarus.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Studies using biological body fluids of patients were carried out on the basis of the “Rules and norms of humane treatment of biological research objects” and were approved at a meeting of the Committee on Biomedical Ethics (protocol no. 2 dated October 4, 2021) of the Belarusian State Medical University. All study participants gave informed consent to participate in the study and use their biological samples in it.
Additional information
Translated by A. Medvedev
Rights and permissions
About this article
Cite this article
Murashka, D.I., Tahanovich, A.D., Kauhanka, M.M. et al. Diagnostic Efficiency of Determining CXCR1, CXCR2 and Hyaluronic Acid in Blood of Patients with Non-Small Cell Lung Cancer. Biochem. Moscow Suppl. Ser. B 16, 45–53 (2022). https://doi.org/10.1134/S1990750822010073
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990750822010073