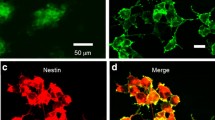
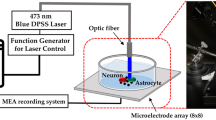
Abstract—
In the central nervous system of mammals, there are specialized areas, known as neurogenic niches, in which neurogenesis is observed in the postnatal period. It is believed that astrocytes in the composition of neurogenic niches play a significant role in the regulation of neurogenesis, and therefore they are considered as a promising “target” for the possible control of neurogenesis, including the use of optogenetics. In the framework of this study, we have formed an in vitro model of a neurogenic niche, consisting of cerebral endothelial cells, astrocytes, and neurospheres. Astrocytes in the neurogenic niche model expressed channelrhodopsin ChR2 and underwent photoactivation. The effect of photoactivated astrocytes on the expression profile of neurogenic niche cells was evaluated using methods of immunocytochemical analysis. It was found that intact astrocytes in the composition of the neurogenic niche promoted neuronal differentiation of stem cells, as well as the activation of astroglia expressing photosensitive proteins, changed expression of molecules characterized by intercellular interactions of pools of resting and proliferating cells in the composition of the neurogenic niche with the participation of NAD+ (Cx43, CD38, CD157), lactate (MCT1). The registered changes reflect: (i) impaired paracrine interactions between two subpopulations of cells, one of which acts as a source of NAD+, and another one is a consumer of NAD+ required for processes of the intracellular signal transduction; (ii) changes in the mechanisms of lactate transport due to aberrant expression of the lactate transporter MCT1 in cells forming a pool of cells developing along the neuronal path of differentiation (but not neuronal stem cells). In general, during photostimulation of niche astrocytes, the total proliferative activity increases mainly due to neural progenitor cells, but not neural stem cells. Thus, optogenetic activation of astrocytes can become a promising tool for controlling the activity of neurogenesis processes and the formation of a local proneurogenic microenvironment in an in vitro model of the neurogenic niche.




Similar content being viewed by others
REFERENCES
Butti, E., Cusimano, M., Bacigaluppi, M., and Martino, G., Front. Neurosci., 2014, vol. 8, pp. 1–11. https://doi.org/10.3389/fnins.2014.00092
Zhao, C., Deng, W., and Gage, F.H., Cell, 2008, vol. 132, no. 4, pp. 645–660. https://doi.org/10.1016/j.cell.2008.01.033
Osipova, E.D., Semyachkina-Glushkovskaya, O.V., Morgun, A.V., Pisareva, N.V., Malinovskaya, N.A., Boytsova, E.B., Pozhilenkova, E.A., Belova, O.A., Salmin, V.V., Taranushenko, T.E., Noda, M., and Salmina A.B., Rev. Neurosci., 2018, vol. 29, no. 5, pp. 567–591. https://doi.org/10.1515/revneuro-2017-0092
Yamazaki, Y. and Kanekiyo, T., Int. J. Mol. Sci., 2017, vol. 9, no. 18, 1965. https://doi.org/10.3390/ijms18091965
Gubert, F., Zaverucha-do-Valle, C., Pimentel-Coelho, P.M., Mendez-Otero, R., and Santiago, M.F., Brain Res., 2009, vol. 1258, pp. 43–52. https://doi.org/10.1016/j.brainres.2008.12.021
Cho, W.-H., Barcelon, E., and Lee, S.J., Exp. Neurobi-ol., 2016, vol. 5, no. 25, 197. https://doi.org/10.5607/en.2016.25.5.197
Gourine, A.V., Kasymov, V., Marina, N., Tang, F., Figueiredo, M.F., Lane, S., Teschemacher, A.G., Spyer, K.M., Deisseroth, K., and Kasparov, S., Science, 2010, vol. 329, no. 5991, pp. 571–575. https://doi.org/10.1126/science.1190721
Figueiredo, M.F., Lane, S., Stout, R.F., Liu, B., Parpura, V., Teschemacher, A.G., and Kasparov, S., Cell Calcium, 2014, vol. 56, no. 3, pp. 208–214. https://doi.org/10.1016/j.ceca.2014.07.007
Khilazheva, E.D., Boytsova, E.B., Pozhilenkova, E.A., Solonchuk, Yu.R., and Salmina, A.B., Cell Tiss. Biol., 2015, vol. 9, pp. 447–451. https://doi.org/10.1134/S1990519X15060048
Liu, Y., Xue, Q., Tang, Q., Hou, M., Qi, H., Chen, G., Chen, W., Zhang, J., Chen, Y., and Xu, X., Microvasc. Res., 2013, vol. 90, pp. 199–205. https://doi.org/10.1016/j.mvr.2013.08.004
Bai, X. and Bosnjak, Z.J., Int. J. Clin. Anesthesiol., 2013, vol. 1, 1002. PMID: 24971394.
Salmina, A.B., Inzhutova, A.I., Morgun, A.V., Okuneva, O.S., Malinovskaya, N.A., Lopatina, O.L., Petrova, M.M., Taranushenko, T.E., Fursov, A.A., and Kuvacheva, N.V., Vestnik Rossiiskoi Akademii Meditsinskikh Nauk, 2012, no. 10, pp. 29–37.
Salmina, A.B., Kuvacheva, N.V., Morgun, A.V., Komleva, Y.K., Pozhilenkova, E.A., Lopatina, O.L., Gorina, Y.V., Taranushenko, T.E., and Petrova, L.L., Int. J. Biochem. Cell Biol., 2015, vol. 64, pp. 174–184. https://doi.org/10.1016/j.biocel.2015.04.005
Salmina, A.B., Morgun, A.V., Kuvacheva, N.V., Lopatina, O.L., Komleva, Y.K., Malinovskaya, N.A., and Pozhilenkova, E.A., Rev. Neurosci., 2014, vol. 25, no. 1, pp. 97–111. https://doi.org/10.1515/revneuro-2013-0044
Morgun, A.V., Kuvacheva, N.V., Khilazheva, E.D., and Salmina, A.B., Sibirskoe Meditsinskoe Obozrenie, 2014, vol. 90, no. 6, pp. 1–5.
Funding
The study was performed within the State Assignment for Research, Ministry of Public Health of Russian Federation (2018−2020) “New technologies for the management of neurogenesis and angiogenesis in the brain.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Authors declare that they have no conflicts of interest. Experiments on animals were carried out in accordance with generally accepted ethical international standards in compliance with the principles of humanity set out in the Directive of the European Community (2010/63/EU), and the requirements of the order of the Ministry of Health of the Russian federation no. 267 of June 19, 2003 “On the approval of the rules of laboratory practice in the Russian Federation.” The study was approved at a meeting of the Bioethical Commission for animals research at the Local Ethics Committee of the prof. V.F. Voino-Yasenetsky Krasnoyarsk State Medical University (protocol no. 4 dated May 16, 2016).
Additional information
Translated by A. Medvedev
Abbreviations used: AVV—adenoviral vector; BBB—blood-brain barrier; ChR2—channelrhodopsin-2; CPE—cytopathic effect; Cx43—connexin 43; GFAP—glial acidic fibrillar protein; GPR81—lactate receptor; MCT1—monocarboxylate transporter 1; Nestin—stem cell marker; NPC—neural progenitor cells; NSC—neural stem cells; PCNA—marker of proliferating cells.
Rights and permissions
About this article
Cite this article
Khilazheva, E.D., Morgun, A.V., Boytsova, E.B. et al. Features of the In Vitro Expression Profile of Hippocampal Neurogenic Niche Cells during Optogenetic Stimulation. Biochem. Moscow Suppl. Ser. B 15, 224–231 (2021). https://doi.org/10.1134/S1990750821030057
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990750821030057