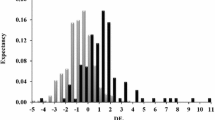
Abstract
Peptic ulcers are the most frequent side effect of therapy with non-steroidal anti-inflammatory drugs (NSAIDs). Good experimental evidence exists that pathogenesis of peptic ulcers cannot be attributed only to inhibition of cyclooxygenases. The knowledge about other molecular mechanisms of drug action associated with development of peptic ulcers could be useful for design of new safer NSAIDs. However, considerable time and material resources are needed for corresponding experimental studies. For simplification of the experimental search, we have developed an approach for in silico identification of putative molecular mechanisms of drug actions associated with their side effects. We have generated a data set of 85 NSAIDs, which includes information about their structures and side effects. Unknown molecular mechanisms of action of these NSAIDs were evaluated by the computer program PASS (Prediction of Activity Spectra for Substances) predicting more than 3000 molecular mechanisms of action based on structural formula of sub-stances. Statistically significant associations have been found between predicted molecular mechanisms of action and development of peptic ulcers. Twenty six molecular mechanisms of action probably associated with development of peptic ulcers have been found: two of them were known previously and 24 were quite new. Analyzing Gene Ontology data, data on signal and metabolic pathways, and available MEDLINE publication data, we proposed hypotheses on the role of 10 molecular mechanisms of action in the pathogenesis of peptic ulcer.
Similar content being viewed by others
References
Al Mofleh, I.A. and Al Rashed, R.S., Saudi J. Gastroenterol., 2007, vol. 13, no. 3, pp. 107–113.
Musumba, C., Pritchard, D.M., and Pirmohamed, M., Aliment. Pharmacol. Ther., 2009, vol. 30, pp. 517–531.
Suleyman, H., Albayrak, A., Bilici, M., Cadirci, E., and Halici, Z., Inflammation, 2010, vol. 33, no. 4, pp. 224–234.
Salvo, F., Fourrier-Réglat, A., Bazin, F., Robinson, P., Riera-Guardia, N., Haag, M., Caputi, A.P., Moore, N., Sturkenboom, M.C., and Pariente, A., Clin. Pharmacol. Ther., 2011, vol. 89, pp. 855–866.
Schubert, M.L., Curr. Opin. Gastroenterol., 2000, vol. 16, pp. 463–468.
Whitebread, S., Hamon, J., Bojanic, D., and Urban, L., Drug Discov. Today, 2005, vol. 10, pp. 1421–1433.
Filimonov, D.A. and Poroikov, V.V., Rus. Khim. Zhurn., 2006, vol. 50, no. 2, pp. 66–75.
Filimonov, D.A. and Poroikov, V.V., in Chemoinformatics Approaches to Virtual Screening, Varnek, A. and Tropsha, A., Eds., Cambridge (UK): RSC Publishing, 2008, pp. 182–216.
Vogt, A., Tamura, K., Watson, S., and Lazo, J.S., J. Pharmacol. Exp. Ther., 2000, vol. 294, pp. 1070–1075.
Mitchell, D.A., Morton, S.U., Fernhoff, N.B., and Marletta, M.A., Proc. Natl. Acad. Sci. USA, 2007, vol. 104, no. 28, pp. 11609–11614.
Watson, W.H., Yang, X., Choi, Y.E., Jones, D.P., and Kehrer, J.P., Toxicol. Sci., 2004, vol. 78, no. 1, pp. 3–14.
Pallis, M., Bradshaw, T.D., Westwell, A.D., Grundy, M., Stevens, M.F., and Russell, N., Biochem. Pharmacol., 2003, vol. 66, pp. 1695–1705.
Tan, A., Nakamura, H., Kondo, N., Tanito, M., et al., Free Radic. Res., 2007, vol. 41, pp. 861–869.
Peskar, B.M., Ehrlich, K., Schuligoi, R., and Peskar, B.A., Pharmacology, 2009, vol. 84, no. 5, pp. 294–299.
Hernandez, D.E., Walker, C.H., and Mason, G.A., Life Sci., 1988, vol. 42, pp. 1757–1764.
Samuels, M.H., Pillote, K., Asher, D., and Nelson, J.C., J. Clin. Endocrinol. Metab., 2003, vol. 88, pp. 5710–5716.
Filaretova, L., Bagaeva, T., and Makara, G.B., Life Sci., 2002, vol. 71, pp. 2457–2468.
Maruyama, K., Okazaki, I., Arai, M., Kurose, I., Komatsu, H., Nakamura, M., and Tsuchiya, M., J. Gastroenterol., 1995, vol. 30, no. 3, pp. 301–309.
Bingle, C.D., Craig, R.W., Swales, B.M., Singleton, V., Zhou, P., and Whyte, M.K., J. Biol. Chem., 2000, vol. 275, pp. 22136–22146.
Kim, N., Yoo, J.C., Han, J.Y., Hwang, E.M., Kim, Y.S., Jeong, E.Y., Sun, C.H., Yi, G.S., Roh, G.S., Kim, H.J., Kang, S.S., Cho, G.J., Park, J.Y., and Choi, W.S., J. Cell. Physiol., 2012, vol. 227, pp. 1157–1167.
Ong, C.C., Jubb, A.M., Haverty, P.M., Zhou, W., Tran, V., Truong, T., Turley, H., O’Brien, T., Vucic, D., Harris, A.L., Belvin, M., Friedman, L.S., Black-wood, E.M., Koeppen, H., Hoeflich, K.P., Proc. Natl. Acad. Sci. USA, 2011, vol. 108, pp. 7177–7182.
Hamel, E., Biopolymers, 2002, vol. 66, no. 3, pp. 142–160.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © S.M. Ivanov, A.A. Lagunin, A.V. Zakharov, D.A. Filimonov, V.V. Poroikov, 2013, published in Biomeditsinskaya Khimiya.
Rights and permissions
About this article
Cite this article
Ivanov, S.M., Lagunin, A.A., Zakharov, A.V. et al. Computer search for molecular mechanisms of ulcerogenic action of non-steroidal anti-inflammatory drugs. Biochem. Moscow Suppl. Ser. B 7, 40–45 (2013). https://doi.org/10.1134/S199075081301006X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S199075081301006X