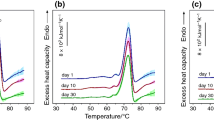
Abstract
Changes in physical and chemical factors appeared in response to freeze-thawing and low temperature storage of biological samples can result in impairments of protein structures. Spontaneous and diamide-induced protein aggregation of placenta blood serum stored at −20 and −196°C up to 2 years has been investigated by SDS-PAGE. It was shown that storage of placental blood serum at low temperatures did not cause any quantitative and qualitative changes in fraction distribution of proteins denatured by SDS compared with native (unfrozen) samples. Application of β-mercaptoethanol revealed that during freeze-thawing placental blood serum proteins did not form spontaneous aggregates cross-linked by disulphide bridges. Oxidation of amino acid sulfhydryl groups induced by diamide and accompanied by formation of high molecular aggregates was a reasonably effective approach for indirect assessment of structural changes in protein molecules induced by low temperatures. In the samples exposed to low temperature storage protein aggregation induced by 4 mM diamide was significantly higher than in native serum. The structural changes in serum proteins caused by low temperatures and recognized by discrepant susceptibility to diamide-induced protein aggregate formation did not depend on temperature (−20 and −196°C) and time-length of storage (2 years and 3 weeks). These changes do reflect protein reaction to freeze-thawing processes and could originate from ice crystal formation which takes place in unprotected media.
Similar content being viewed by others
References
Wan, G., Yu, S., and Liu, J., J. Chung Hua Fu Chan Ko Tsa Chih., 1998, vol. 33, pp. 720–721.
Schneider, H. and Maiek, A., J. Perinat. Med., 1995, vol. 23, pp. 71–76.
Kyung-Chul Yoon, Hwan Heo, In-Yong Jeong, Yeoung Geol Park, Kor. J. Ophthalmol., 2005, vol. 19, pp. 174–178.
Sagawa, N., Yura, S., Itoh, H., Kakui, K., Takemura, M., Nuamah, M.A., Ogawa, Y., Masuzaki, H., Nakao, K., and Fujii, S., J. Endocrine, 2002, vol. 19, pp. 65–71.
Seifarth, C.C., Miertschischk, J., Hahn, E.G., and Hensen, J., Clin. Chem. Lab. Med., 2004, vol. 42, pp. 927–932.
Gislefoss, R.E., Grimsrud, T.K., Morkrid, L., Scand. J. Clin. Lab. Invest., 2008, vol. 68, pp. 402–409.
Hostmark, A.T., Glattre, E., and Jellum, E., Scand. J. Clin. Lab. Invest., 2001, vol. 61, pp. 443–447.
Lev, E.I., Hendler, I., Siebner, R., Tashma, Z., Wiener, M., and Tur-Kaspa, I., Enzyme Protein, 1994–1995, vol. 48, pp. 238–242.
Cray, C., Rodriguez, M., Zaias, J., and Altman, N.H., J. Am. Assoc. Lab. Anim. Sci., 2009, vol. 48, pp. 202–204.
Muller, F., Tyler, J.W., Parish, S.M., Johnson, K.A., Krytenberg, D.S., and Wilson, L.K., J. Am. Vet. Res., 1997, vol. 58, pp. 354–355.
Ito, Y., Nakachi, K., Imai, K., Hashimoto, S., Watanabe, Y., Inaba, Y., Tamakoshi, A., and Yoshimura, T., J. Epidemal., 2005, Suppl. 1, pp. 67–73.
Shurygin, D.Ya., Kholodnyi, A.Ya., Mazurov, V.I., and Yakovlev, V.A., Probl. Endokrinol. (Moscow), 1981, vol. 27, no. 3, pp. 32–35.
Gao, Y.C., Yuan, Z.B., Yang, Y.D., and Lu, H.K., Scand. J. Clin. Lab. Invest., 2007, vol. 67, pp. 741–747.
Siennicka, J., Laskowska, A., Trzcin-ska, A., Med. Dosw. Mikrobiol., 2010, vol. 62, pp. 281–283.
Huizenga, J.R., van der Belt, K., Gips, C.H.J., Clin. Chem. Clin. Biochem., 1985, vol. 23, pp. 283–285.
Natalello, A., Santarella, R., Doglia, S.M., de Marco, A., Protein Expr. Purif., 2008, vol. 58, pp. 356–361.
Eckhardt, B.M., Oeswein, J.Q., and Bewley, T.A., Pharm. Res., 1991, vol. 8, pp. 1360–1364.
Osterman, L.A., Metody issledovaniya belkov i nucleinovykh kislot. Elektroforez i ultratsentrifugirovanie (Methods for Studies of Proteins and Nucleic Acids), Moscow: Nauka, 1981.
Bradford, M.M., Anal. Biochem., 1976, vol. 72, pp. 248–254.
Bailey, N., Statisticheskie metody v biologii (Statistical Methods in Biology), Moscow: Mir, 1963.
Pieper, R., Gatlin, C.L., Makusky, A.J., Russo, P.S., Schatz, C.R., Miller, S.S., Su, Q., McGrath, A.M., Estock, M.A., Parmar, P.P., Zhao, M., Huang, S.T., Zhou, J., Wang, F., Esquer-Blasco, R., Anderson, N.L., Taylor, J., and Steiner, S., Proteomics, 2003, vol. 3, pp. 1345–1364.
Bhatnagar, B.S., Bogner, R.H., and Pikal, M.J., Pharm. Dev. Technol., 2007, vol. 12, pp. 505–523.
Chang, B.S., Kendrick, B.S., and Carpenter, J.F., J. Pharm. Sci., 1996, vol. 85, pp. 1325–1330.
Lin, J.J., Meyer, J.D., Carpenter, J.F., and Manning, M.C., Pharm. Res., 2000, vol. 17, pp. 391–396.
Hawe, A., Kasper, J.C., Friess, W., and Jiskoot, W., Eur. J. Pharm. Sci., 2009, vol. 38, pp. 79–87.
Strambini, G.B. and Gabellieri, E., Biophys J., 1996, vol. 70, pp. 971–976.
Gonnelli, M. and Strambini, G.B., Biochemistry, 1995, vol. 34, pp. 13847–13857.
Gonnelli, M. and Strambini, G.B., Biophys. Chem., 1986, vol. 24, pp. 161–167.
Gabellieri, E. and Strambini, G.B., Biophys. J., 2003, vol. 85, pp. 3214–3220.
Deuticke, B., Poser, B., Lütkemeier, P., and Haest, C.W., Biochim. Biophys. Acta., 1983, vol. 731, pp. 196–210.
Fischer, T.M., Haest, C.W., Stöhr, M., Kamp, D., and Deuticke, B., Biochem. Biophys. Acta., 1978, vol. 510, pp. 270–282.
Zemlyanskikh, N.G. and Denisova, O.N., Biofizika, 2009, vol. 54, pp. 693–703.
Turell, L., Carballal, S., Botti, H., Radi, R., and Alvarez, B., Braz. J. Med. Biol. Res., 2009, vol. 42, pp. 305–311.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © O.V. Falko, N.G. Zemlianskykh, O.V. Lipina, O.S. Prokopyuk, 2012, published in Biomeditsinskaya Khimiya.
Rights and permissions
About this article
Cite this article
Falko, O.V., Zemlianskykh, N.G., Lipina, O.V. et al. Modification of placental blood serum proteins induced by low temperatures. Biochem. Moscow Suppl. Ser. B 6, 192–200 (2012). https://doi.org/10.1134/S1990750812020047
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990750812020047