Abstract
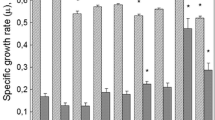
The effects of five new derivatives of 2,6-dialkyl-4-propylphenol containing different ionogenic groups (-SO3Na, -S-SO3Na, -S-(NH2)2Cl) in the para position on survival of the E. coli AB1157 strain and its isogenic strain deficient in repair enzyme genes have been investigated in the presence and in the absence of hydrogen peroxide. Survival of cells treated with hydrogen peroxide was markedly increased in the presence of sodium (3-(3,5-dimethyl-4-hydroxyphenyl)propyl)-1-sulphonate (C1). Replacement of methyl substituents at ortho-position of C1 for tert-butyl or cyclohexyl groups decreased cell protection against exogenous hydrogen peroxide. Among 2,6-di-tert-buthylphenol the compound with the thiosulphonate group demonstrated properties similar to its sulphonate analog, whereas 3 mM isothiuronium chloride completely suppressed cell growth both in the absence and in the presence of hydrogen peroxide. Thus, among the tested compounds C1 may be considered as the most promising antioxidant.
Similar content being viewed by others
Abbreviations
- AO:
-
antioxidant
- CFU:
-
colony forming unit
- ROS:
-
reactive oxygen species
References
Sies, H., Oxidative Stress, London: Academic Press, 1985, pp. 1–8.
Sies, H., Amer. J. Med., 1991, vol. 91, pp. 313–323.
Seyfulla, R.D. and Borisova, I.G., Farmakol. Toksikol., 1990, no. 6, pp. 3–10.
Men’schikova, E.B., Lankin, V.Z. Zenkov, N.K., Bondar’, I.A., Krugovykh, I.F., and Trufakin, V.A., Okislitel’nyi stress. Prooksidanty i antioksidanty (Oxidative Stress. Prooxidants and Antioxidants), Moscow: Slovo, 2006.
Vol’skii, N.N., Kashlakova, N.V., and Kozlov, V.A., Tsitologiya, 1988, vol. 30, pp. 895–902.
Richards, D.M.S., Dean, R.T., and Jessup, W., Biochim. Biophys. Acta, 1988, vol. 946, pp. 281–288.
Drapier, J.-C. and Hibbs, J.B., J. Immunol., 1988, vol. 140, pp. 2829–2838.
Hoffman, M.E., Mello-Filho, A.C., and Meneghini, R., Biochim. Biophys. Acta, 1984, vol. 781, pp. 234–238.
Harman, D., Mutat. Res., 1992, vol. 275, pp. 257–266.
Beckman, K.B. and Ames, B.N., Physiol. Rev., 1998, vol. 78, pp. 547–581.
Grollman, A.P. and Moriya, M., Trends Genet., 1993, vol. 9, pp. 246–249.
Halliwell, B., Lancet, 1994, vol. 344, pp. 721–724.
Zhuravlev, A.I., Bioantiokisliteli v regulyatsii metabolisma v norme i patologii, (Bioantioxidants in the regulation of metabolism in norm and pathology), Moscow: Nauka, 1982, pp. 3–37.
Bast, A. and Haenen, G.R., Antioxidants in Therapy and Preventive Medicine, N.-Y.: Plenum Press, 1990, pp. 111–116.
Munday, R. and Winterboume, C.C., Biochem. Pharmacol., 1989, vol. 38, pp. 4349–4352.
Wengel, A., Enzymes — Tools and Targets, Basel: Karget, 1988, pp. 161–167.
Kenya, M.V., Lukash, A.I., Gus’kov, E.P., Usp. Sovr. Biol., 1993, vol. 113, pp. 456–470.
Abramova, Zh.I. and Oksengendler, G.I., Chelovek i protivookislitel’nye veshchestva, (Man and Antioxidant Substances), Leningrad; Nauka, 1985.
Beyer, R.E., Free Radical Biol. Med., 1990, vol. 8, pp. 545–565.
Roginskii, V.A., Fenol’nyie antioksidanty: reaktsionnaya sposobnost’ i effektivnost’ (Phenolic Antioxidants: Reactivity and Effectiveness), Moscow: Nauka, 1988.
Mickle, D.A.G., Li, R.K., Weisel, R.D., Tumiati, L.C., and Wu, T.W., J. Mol. Cell. Cardiol., 1990, vol. 22, pp. 1297–1304.
Voskresenskii, O.N., Zhutaev, I.A., Bobyrev, V.N., and Bezuglyi, Yu.V., Vopr. Med. Khim., 1982, no. 1, pp. 14–27.
Sokolovskii, V.V., Vopr. Med. Khim., 1988, no. 6, pp. 3–10.
Maron, D.M. and Ames, B.N., Mutat. Res., 1983, vol. 113, pp. 173–215.
Quillardet, P. and Hofnung, M., Mutat. Res., 1985, vol. 147, pp. 65–78.
Asad, N.R., CEB de Almedia, LMBO Asad, Felzeswalb, I., and Leitao, A.C., Biochimie, 1995, vol. 77, pp. 262–264.
Gomes, E.M., Souto, P.R., and Felzeswalb, I., Mutat. Res., 1996, vol. 367, pp. 203–208.
Imlay, J., and Linn, S., Science, 1988, vol. 240, pp. 640–642, 1302–1309.
Asad, N.R. and Leitao, A.C., J. Bacteriol., 1991, vol. 173, pp. 2562–2568.
Kemeleva, E.A., Vasyunina, E.A., Sinitsyna, O.I., Khomchenko, A.S., Gross, M.A., Kandalintseva, N.V., Prosenko, A.E., and Nevinsky, G.A., Bioorgan. Khim., 2008, vol. 34, pp. 499–509.
DeWitt, S.K. and Adelbeg, E.A., Genetics, 1962, vol. 47, pp. 577–585.
Oleinick, A.S., Kuprina, T.S., Pevneva, N.Yu., Markov, A.F., Kandalintseva, N.V., Prosenko, A.E., and Grigor’ev, I.A., Izv. Akad. Nauk, Ser. Khim., 2007, vol. 6, pp. 1094–1101.
Kandalintseva, N.V., Prosenko, A.E., Dyubchenko, O.I., and Stoyanov, E.S., Zh. Org. Khim., 2001, vol. 9, pp. 1317–1320.
Miller, J., Eksperimenty v molekulyarnoi genetike, (Experiments in Molecular Genetics), Mir: Moscow, 1976.
Bhatnagar, S.K. and Bessman, M.J., J. Biol. Chem., 1988, vol. 263, pp. 8953–8957.
Miller, J.H. and Michael, M., Gene, 1996, vol. 179, pp. 129–132.
Lin, J.J. and Sancar, A., Bochemistry, 1989, vol. 28, pp. 7979–7984.
Oleinik, A.S., Pevneva, N.Yu., Kandalintseva, N.V., Prosenko, A.E., Khoschenko, O.M., and Dushkin, M.I., Khimiya v Interesakh Ustoichivogo Razvitiya, 2008, vol. 5, pp. 559–564.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © U.N. Rotskaya, L.P. Ovchinnikova, E.A. Vasunina, O.I. Sinitsina, N.V. Kandalintseva, E.A. Prosenko, G.A. Nevinsky, 2010, published in Biomeditsinskaya Khimiya.
Rights and permissions
About this article
Cite this article
Rotskaya, U.N., Ovchinnikova, L.P., Vasunina, E.A. et al. Evaluation of cytotoxicity and efficiency of antioxidant protection of hydrophilic derivatives of 2,4,6-trialkylphenols in Escherichia coli cells. Biochem. Moscow Suppl. Ser. B 4, 377–382 (2010). https://doi.org/10.1134/S1990750810040098
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990750810040098