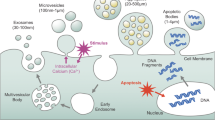
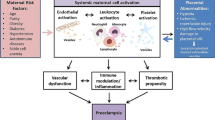
Abstract
Extracellular vesicles are lipid bilayer membrane structures without nuclei that are released from various cells as a result of physiological and metabolic changes. They play an important role in intercellular communication through the transfer of a wide range of bioactive molecules, contributing to the regulation of various physiological and pathological processes. Extracellular vesicles may have procoagulant properties due to the presence of phosphatidylserine, which accelerates coagulation reactions, on the outer layer of the membrane, as well as the expression of tissue factor, which activates coagulation along the external pathway, on the surface of some vesicles. A large number of clinical and experimental studies have shown that in various pathologies and specific physiological conditions, including pregnancy, the concentration of extracellular vesicles significantly exceeds that in healthy volunteers, which could theoretically be a factor in the development of hypercoagulable states This review focuses on describing the procoagulant properties of extracellular vesicles of various origins in normal and pathological pregnancy.


Similar content being viewed by others
REFERENCES
Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., Ayre D.C., Bach J.M., Bachurski D., Baharvand H., Balaj L., et al. 2018. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 7 (1), 1535750. https://doi.org/10.1080/20013078.2018.1535750
Yáñez-Mó M., Siljander P.R., Andreu Z., Zavec A.B., Borràs F.E., Buzas E.I., Buzas K., Casal E., Cappello F., Carvalho J., Colás E., Cordeiro-da Silva A., Fais S., Falcon-Perez J.M., Ghobrial I.M., et al. 2015. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 4 (2015), 27066. https://doi.org/10.3402/jev.v4.27066
Colombo M., Raposo G., Théry C. 2014. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30, 255–289. https://doi.org/10.1146/annurev-cellbio-101512-122326
Rojalin T., Phong B., Koster H.J., Carney R.P. 2019. Nanoplasmonic approaches for sensitive detection and molecular characterization of extracellular vesicles. Front. Chem. 7, 279. https://doi.org/10.3389/fchem.2019.00279
Raposo G., Stoorvogel W. 2013. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 200 (4), 373–383. https://doi.org/10.1083/jcb.201211138
Bebelman M.P., Smit M.J., Pegtel D.M., Baglio S.R. 2018. Biogenesis and function of extracellular vesicles in cancer. Pharmacol. Ther. 188, 1–11. https://doi.org/10.1016/j.pharmthera.2018.02.013
Cretoiu D., Xu J., Xiao J., Cretoiu S.M. 2016. Telocytes and their extracellular vesicles-evidence and hypotheses. Int. J. Mol. Sci. 17 (8), 1322. https://doi.org/10.3390/ijms17081322
Wickman G., Julian L., Olson M.F. 2012. How apoptotic cells aid in the removal of their own cold dead bodies. Cell Death Differ. 19 (5), 735–742. https://doi.org/10.1038/cdd.2012.25
Tannetta D., Dragovic R., Alyahyaei Z., Southcombe J. 2014. Extracellular vesicles and reproduction–promotion of successful pregnancy. Cell. Mol. Immunol. 11 (6), 548–563. https://doi.org/10.1038/cmi.2014.42
Burnett L.A., Nowak R.A. 2016. Exosomes mediate embryo and maternal interactions at implantation and during pregnancy. Front. Biosci. 8 (1), 79–96. https://doi.org/10.2741/s448
Chiarello D.I., Salsoso R., Toledo F., Mate A., Vázquez C.M., Sobrevia L. 2018. Foetoplacental communication via extracellular vesicles in normal pregnancy and preeclampsia. Mol. Aspects Med. 60, 69–80. https://doi.org/10.1016/j.mam.2017.12.002
Sheller-Miller S., Choi K., Choi C., Menon R. 2019. Cyclic-recombinase-reporter mouse model to determine exosome communication and function during pregnancy. Am. J. Obstet. Gynecol. 221 (5), 502.e1-502.e12. https://doi.org/10.1016/j.ajog.2019.06.010
Han C., Han L., Huang P., Chen Y., Wang Y., Xue F. 2019. syncytiotrophoblast-derived extracellular vesicles in pathophysiology of preeclampsia. Front. Physiol. 10, 1236. https://doi.org/10.3389/fphys.2019.01236
James-Allan L.B., Devaskar S.U. 2021. Extracellular vesicles and their role in gestational diabetes mellitus. Placenta. 113, 15–22. https://doi.org/10.1016/j.placenta.2021.02.012
Menon R, Shahin H. 2021. Extracellular vesicles in spontaneous preterm birth. Am. J. Reprod. Immunol. 85 (2), 139–148. https://doi.org/10.1111/aji.13353
Weiss R., Gröger M., Rauscher S., Fendl B., Eichhorn T., Fischer M.B., Spittler A., Weber V. 2018. differential interaction of platelet-derived extracellular vesicles with leukocyte subsets in human whole blood. Sci. Rep. 8 (1), 6598. https://doi.org/10.1038/s41598-018-25047-x
Arraud N., Linares R., Tan S., Gounou C., Pasquet J.M., Mornet S., Brisson A.R. 2014. Extracellular vesicles from blood plasma: Determination of their morphology, size, phenotype and concentration. J. Thromb. Haemost. 12 (5), 614–627. https://doi.org/10.1111/jth.12554
Koltsova E.M., Sorokina M.A., Pisaryuk A.S., Povalyaev N.M., Ignatova A.A., Polokhov D.M., Kotova E.O., Balatskiy A.V., Ataullakhanov F.I., Panteleev M.A., Kobalava Z.D., Balandina A.N. 2021. Hypercoagulation detected by routine and global laboratory hemostasis assays in patients with infective endocarditis. PLoS One. 16 (12), e0261429. https://doi.org/10.1371/journal.pone.0261429
Combes V., Simon A.C., Grau G.E., Arnoux D., Camoin L., Sabatier F., Mutin M., Sanmarco M., Sampol J., Dignat-George F. 1999. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J. Clin. Invest. 104 (1), 93–102. https://doi.org/10.1172/JCI4985
Dickhout A., Koenen R.R. 2018. Extracellular vesicles as biomarkers in cardiovascular disease; chances and risks. Front. Cardiovasc. Med. 5, 113. https://doi.org/10.3389/fcvm.2018.00113
Dignat-George F., Boulanger C.M. 2011. The many faces of endothelial microparticles. Arterioscler. Thromb. Vasc. Biol. 31(1), 27–33. https://doi.org/10.1161/ATVBAHA.110.218123
Sedgwick A.E., D’Souza-Schorey C. 2018. The biology of extracellular microvesicles. Traffic. 19 (5), 319–327. https://doi.org/10.1111/tra.12558
Thangaraju K., Neerukonda S.N., Katneni U., Buehler P.W. 2020. Extracellular vesicles from red blood cells and their evolving roles in health, coagulopathy and therapy. Int. J. Mol. Sci. 22 (1), 153. https://doi.org/10.3390/ijms22010153
Gamonet C., Desmarets M., Mourey G., Biichle S., Aupet S., Laheurte C., François A., Resch E., Bigey F., Binda D., Bardiaux L., Naegelen C., Marpaux N., Delettre F.A., Saas P., Morel P., Tiberghien P., Lacroix J., Capellier G., Vidal C., Garnache-Ottou F. 2020. Processing methods and storage duration impact extracellular vesicle counts in red blood cell units. Blood Adv. 4 (21), 5527–5539. https://doi.org/10.1182/bloodadvances.2020001658
Jy W., Ricci M., Shariatmadar S., Gomez-Marin O., Horstman L.H., Ahn Y.S. 2011. Microparticles in stored red blood cells as potential mediators of transfusion complications. Transfusion. 51 (4), 886–893. https://doi.org/10.1111/j.1537-2995.2011.03099.x
Giesen P.L.A., Rauch U., Bohrmann B., Kling D., Roqué M., Fallon J.T., Badimon J.J., Himber J., Riederer M.A., Nemerson Y. 1999. Blood-borne tissue factor: Another view of thrombosis. Proc. Natl. Acad. Sci. USA. 96 (5), 2311–2315. https://doi.org/10.1073/pnas.96.5.2311
Reddy E.C., Rand M.L. 2020. Procoagulant phosphatidylserine-exposing platelets in vitro and in vivo. Front Cardiovasc. Med. 7 (15), 15. https://doi.org/10.3389/fcvm.2020.00015
Mackman N. 2009. The many faces of tissue factor. J. Thromb. Haemost. 7, 136–139. https://doi.org/10.1111/j.1538-7836.2009.03368.x
Ruf W., Dorfleutner A., Riewald M. 2003. Specificity of coagulation factor signaling. J. Thromb. Haemost. 1 (7), 1495–1503. https://doi.org/10.1046/j.1538-7836.2003.00300.x
Monroe D.M., Key N.S. 2007. The tissue factor-factor VIIa complex: Procoagulant activity, regulation, and multitasking. J. Thromb. Haemost. 5 (6), 1097–1105. https://doi.org/10.1111/j.1538-7836.2007.02435.x
Butenas S., Orfeo T., Mann K.G. 2009. Tissue factor in coagulation: Which? Where? When? Arterioscler. Thromb. Vasc. Biol. 29 (12), 1989–1996. https://doi.org/10.1161/ATVBAHA.108.177402
Mackman N., Tilley R.E., Key N.S. 2007. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler. Thromb. Vasc. Biol. 27 (8), 1687–1693. https://doi.org/10.1161/ATVBAHA.107.141911
Andrews A.M., Rizzo V. 2016. Microparticle-induced activation of the vascular endothelium requires caveolin-1/caveolae. PLoS One. 11 (2), e0149272. https://doi.org/10.1371/journal.pone.0149272
Shustova O.N., Antonova O.A., Golubeva N.V., Khaspekova S.G., Yakushkin V.V., Aksuk S.A., Alchinova I.B., Karganov M.Y., Mazurov A.V. 2017. Differential procoagulant activity of microparticles derived from monocytes, granulocytes, platelets and endothelial cells: Impact of active tissue factor. Blood Coagul. Fibrinolysis. 28 (5), 373–382. https://doi.org/10.1097/MBC.0000000000000609
Yang A., Chen F., He C., Zhou J., Lu Y., Dai J., Birge R.B., Wu Y. 2017. The procoagulant activity of apoptotic cells is mediated by interaction with factor XII. Front. Immunol. 8, 1188. https://doi.org/10.3389/fimmu.2017.01188
Bretelle F., Sabatier F., Desprez D., Camoin L., Grunebaum L., Combes V., D’Ercole C., Dignat-George F. 2003. Circulating microparticles: A marker of procoagulant state in normal pregnancy and pregnancy complicated by preeclampsia or intrauterine growth restriction. Thromb. Haemost. 89 (3), 486–492. https://doi.org/10.1055/s-0037-1613378
Alijotas-Reig J., Palacio-Garcia C., Farran-Codina I., Zarzoso C., Cabero-Roura L., Vilardell-Tarres M. 2011. Circulating cell-derived microparticles in women with pregnancy loss. Am. J. Reprod. Immunol. 66 (3), 199–208. https://doi.org/10.1111/j.1600-0897.2010.00972.x
Radu C.M., Campello E., Spiezia L., Dhima S., Visentin S., Gavasso S., Woodhams B., Cosmi E., Simioni P. 2015. Origin and levels of circulating microparticles in normal pregnancy: A longitudinal observation in healthy women. Scand. J. Clin. Lab. Invest. 75 (6), 487–495. https://doi.org/10.3109/00365513.2015.1052551
Zhang Y., Zhao C., Wei Y., Yang S., Cui C., Yang J., Zhang J., Qiao R. 2018. Increased circulating microparticles in women with preeclampsia. Int. J. Lab. Hematol. 40 (3), 352–358. https://doi.org/10.1111/ijlh.12796
Alijotas-Reig J., Palacio-Garcia C., Llurba E., Vilardell-Tarres M. 2013. Cell-derived microparticles and vascular pregnancy complications: A systematic and comprehensive review. Fertil. Steril. 99 (2), 441–449. https://doi.org/10.1016/j.fertnstert.2012.10.009
Aharon A., Brenner B. 2011. Microparticles and pregnancy complications. Thromb. Res. 127, S67–S71. https://doi.org/10.1016/S0049-3848(11)70019-6
Burton G.J., Fowden A.L. 2015. The placenta: A multifaceted, transient organ. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 370 (1663), 20140066. https://doi.org/10.1098/rstb.2014.0066
Huppertz B., Kadyrov M., Kingdom J.C.P. 2006. Apoptosis and its role in the trophoblast. Am. J. Obstet. Gynecol. 195 (1), 29–39. https://doi.org/10.1016/j.ajog.2005.07.039
Haider S., Meinhardt G., Saleh L., Kunihs V., Gamperl M., Kaindl U., Ellinger A., Burkard T.R., Fiala C., Pollheimer J., Mendjan S., Latos P.A., Knöfler M. 2018. Self-renewing trophoblast organoids recapitulate the developmental program of the early human placenta. Stem Cell Reports. 11 (2), 537–551. https://doi.org/10.1016/j.stemcr.2018.07.004
Moffett A., Loke C. 2006. Immunology of placentation in eutherian mammals. Nat. Rev. Immunol. 6 (8), 584–594. https://doi.org/10.1038/nri1897
Askelund K.J., Chamley L.W. 2011. Trophoblast deportation part I: review of the evidence demonstrating trophoblast shedding and deportation during human pregnancy. Placenta. 32 (10), 716–723. https://doi.org/10.1016/j.placenta.2011.07.081
Holland O., Kroneis T., El-Heliebi A., McDowell-Hook M., Stone P., Sedlmayr P., Chamley L. 2017. Detection of fetal sex, aneuploidy and a microdeletion from single placental syncytial nuclear aggregates. Fetal Diagn. Ther. 41 (1), 32–40. https://doi.org/10.1159/000445112
Johansen M., Redman C.W., Wilkins T., Sargent I.L. 1999. Trophoblast deportation in human pregnancy—its relevance for pre-eclampsia. Placenta. 20 (7), 531–539. https://doi.org/10.1053/plac.1999.0422
Reverdiau P., Jarousseau A.C., Thibault G., Khalfoun B., Watier H., Lebranchu Y., Bardos P., Gruel Y. 1995. Tissue factor activity of syncytiotrophoblast plasma membranes and tumoral trophoblast cells in culture. Thromb. Haemost. 73 (1), 49–54. https://doi.org/10.1055/s-0038-1653724
Teng Y.C., Lin Q.De., Lin J.H., Ding C.W., Zuo Y. 2009. Coagulation and fibrinolysis related cytokine imbalance in preeclampsia: The role of placental trophoblasts. J. Perinat. Med. 37 (4), 343–348. https://doi.org/10.1515/JPM.2009.060
Aharon A., Brenner B., Katz T., Miyagi Y., Lanir N. 2004. Tissue factor and tissue factor pathway inhibitor levels in trophoblast cells: Implications for placental hemostasis. Thromb. Haemost. 92 (4), 776–786. https://doi.org/10.1160/TH04-01-0033
Lakasing L., Campa J.S., Poston R., Khamashta M.A., Poston L. 1999. Normal expression of tissue factor, thrombomodulin, and annexin V in placentas from women with antiphospholipid syndrome. Am. J. Obstet. Gynecol. 181 (1), 180–189. https://doi.org/10.1016/s0002-9378(99)70457-6
Faulk W.P., Labarrere C.A., Carson S.D. 1990. Tissue factor: Identification and characterization of cell types in human placentae. Blood. 76 (1), 86–96.
Teng Y., Jiang R., Lin Q., Ding C., Ye Z. 2010. The relationship between plasma and placental tissue factor, and tissue factor pathway inhibitors in severe pre-eclampsia patients. Thromb. Res. 126 (1), e41–e45. https://doi.org/10.1016/j.thromres.2010.02.012
Gardiner C., Tannetta D.S., Simms C.A., Harrison P., Redman C.W.G., Sargent I.L. 2011. Syncytiotrophoblast microvesicles released from pre-eclampsia placentae exhibit increased tissue factor activity. PLoS One. 6 (10), e26313. https://doi.org/10.1371/journal.pone.0026313
Ng E.K.O, Leung T.N, Tsui N.B.Y, Lau T.K, Panesar N.S, Chiu R.W.K, Lo Y.M.D. 2003. The concentration of circulating corticotropin-releasing hormone mRNA in maternal plasma is increased in preeclampsia. Clin. Chem. 49 (5), 727–731. https://doi.org/10.1373/49.5.727
Freeman D.J., Tham K., Brown E.A., Rumley A., Lowe G.D., Greer I.A. 2008. Fetal corticotrophin-releasing hormone mRNA, but not phosphatidylserine-exposing microparticles, in maternal plasma are associated with factor VII activity in pre-eclampsia. J. Thromb. Haemost. 6 (3), 421–427. https://doi.org/10.1111/j.1538-7836.2007.02882.x
Goswami D., Tannetta D.S., Magee L.A., Fuchisawa A., Redman C.W.G., Sargent I.L., von Dadelszen P. 2006. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta. 27 (1), 56–61. https://doi.org/10.1016/j.placenta.2004.11.007
Knight M., Redman C.W.G., Linton E.A., Sargent I.L. 1998. Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. BJOG. 105 (6), 632–640. https://doi.org/10.1111/j.1471-0528.1998.tb10178.x
Lok C.A.R, Van Der Post J.A.M, Sargent I.L., Hau C.M., Sturk A., Boer K., Nieuwland R. 2008. Changes in microparticle numbers and cellular origin during pregnancy and preeclampsia. Hypertens. Pregnancy. 27 (4), 344–360. https://doi.org/10.1080/10641950801955733
Huppertz B., Frank H.G., Kingdom J.C., Reister F., Kaufmann P. 1998. Villous cytotrophoblast regulation of the syncytial apoptotic cascade in the human placenta. Histochem. Cell Biol. 110 (5), 495–508. https://doi.org/10.1007/s004180050311
Owens A.P. 3rd, Mackman N. 2012. Microparticles in hemostasis and thrombosis. Circ. Res. 108 (10), 1284–1297. https://doi.org/10.1161/CIRCRESAHA.110.233056
Fazel A., Vincenot A., Malassiné A., Soncin F., Gaussem P., Alsat E., Evain-Brion D. 1998. Increase in expression and activity of thrombomodulin in term human syncytiotrophoblast microvilli. Placenta. 19 (4), 261–268. https://doi.org/10.1016/s0143-4004(98)90057-1
Lanir N., Aharon A., Brenner B. 2003. Haemostatic mechanisms in human placenta. Best Pract. Res. Clin. Haematol. 16 (2), 183–95. https://doi.org/10.1016/s1521-6926(02)00098-1
Štok U., Čučnik S., Sodin-Šemrl S., Žigon P. 2021. Extracellular vesicles and antiphospholipid syndrome: state-of-the-art and future challenges. Int. J. Mol. Sci. 22 (9), 4689. https://doi.org/10.3390/ijms22094689
Goulopoulou S., Davidge S.T. 2015. Molecular mechanisms of maternal vascular dysfunction in preeclampsia. Trends Mol. Med. 21 (2), 88–97. https://doi.org/10.1016/j.molmed.2014.11.009
Tannetta D.S., Dragovic R.A., Gardiner C., Redman C.W., Sargent I.L. 2013. Characterisation of syncytiotrophoblast vesicles in normal pregnancy and pre-eclampsia: Expression of Flt-1 and endoglin. PLoS One. 8 (2), e56754. https://doi.org/10.1371/journal.pone.0056754
Lok C.A.R., Böing A.N., Sargent I.L., Sooranna S.R., van der Post J.A.M., Nieuwland R., Sturk A. 2008. Circulating platelet-derived and placenta-derived microparticles expose Flt-1 in preeclampsia. Reprod. Sci. 15 (10), 1002–1010. https://doi.org/10.1177/1933719108324133
Cronqvist T., Tannetta D., Mörgelin M., Belting M., Sargent I., Familari M., Hansson S.R. 2017. Syncytiotrophoblast derived extracellular vesicles transfer functional placental miRNAs to primary human endothelial cells. Sci. Rep. 7 (1), 4558. https://doi.org/10.1038/s41598-017-04468-0
Tannetta D.S., Hunt K., Jones C.I., Davidson N., Coxon C.H., Ferguson D., Redman C.W., Gibbins J.M., Sargent I.L., Tucker K.L. 2015. Syncytiotrophoblast extracellular vesicles from pre-eclampsia placentas differentially affect platelet function. PLoS One. 10 (11), e0142538. https://doi.org/10.1371/journal.pone.0142538
Tannetta D., Masliukaite I., Vatish M., Redman C., Sargent I. 2017. Update of syncytiotrophoblast derived extracellular vesicles in normal pregnancy and preeclampsia. J. Reprod. Immunol. 119, 98–106. https://doi.org/10.1016/j.jri.2016.08.008
Funding
This work was supported by grants from the President of the Russian Federation for E.M. Koltsova (project no. MK-432.2020.7, agreement 075-15-2020-181) and N.A. Podoplelov (project no. MK-6271.2021.1.4, agreement 075-15-2021-413) and a scholarship from the President of the Russian Federation for A.A. Martyanov (project no. SP-2675.2019.4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by E. Koltsova
Rights and permissions
About this article
Cite this article
Koltsova, E.M., Martyanov, A.A. & Podoplelova, N.A. Procoagulant Properties of Extracellular Vesicles in Normal and Pathological Pregnancy. Biochem. Moscow Suppl. Ser. A 17, 12–19 (2023). https://doi.org/10.1134/S1990747822060071
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747822060071