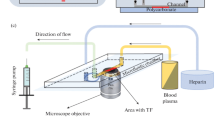
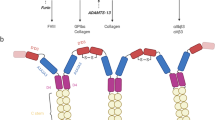
Abstract—
Blood coagulation and fibrinolysis systems are enzymatic cascades in blood plasma that control formation and dissolution of a fibrin clot, respectively. However, critical processes in both systems occur on specialized scaffolds but not in the liquid phase. These scaffolds are two- or three-dimensional matrices that provide special conditions for biochemical reactions. The following fundamental categories of scaffolds can be distinguished: (a) phospholipid membranes enriched with phosphatidylserine provided by a procoagulant subpopulation of activated platelets, as well as damaged endothelium; membranes of apoptotic bodies in atherosclerotic plaque; lipoproteins and plasma microvesicles; (b) complex of fibrin and extracellular matrix proteins, which is associated with platelets and is the leading scaffold for pro- and anti-fibrinolytic processes; (c) polymers containing phosphate groups, including platelet polyphosphates and neutrophil extracellular traps. For some of these scaffolds, there are speculations about their physiological significance and physical meaning, while the role of others seems mysterious or at least pathophysiological. Herein we consider existing ideas about the roles and mechanisms of the involvement of these scaffolds in haemostasis and thrombosis.


Similar content being viewed by others
REFERENCES
Mann K.G., Orfeo T., Butenas S., Undas A., Brummel-Ziedins K. 2009. Blood coagulation dynamics in haemostasis. Hamostaseologie. 29 (1), 7–16.
Shibeko A.M., Panteleev M.A. 2016. Untangling the complexity of blood coagulation network: Use of computational modelling in pharmacology and diagnostics. Brief Bioinform. 17 (3), 429–439.
Panteleev M.A., Andreeva A.A., Lobanov A.I. 2020. Differential drug target selection in blood coagulation: What can we get from computational systems biology models? Curr. Pharm. Des. 26 (18), 2109–2115.
Panteleev M.A., Balandina A.N., Lipets E.N., Ovanesov M.V., Ataullakhanov F.I. 2010. Task-oriented modular decomposition of biological networks: Trigger mechanism in blood coagulation. Biophys. J. 98 (9), 1751–1761.
Shibeko A.M., Chopard B., Hoekstra A.G., Panteleev M.A. 2020. Redistribution of TPA fluxes in the presence of PAI-1 regulates spatial thrombolysis. Biophys. J. 119 (3), 638–651.
Panteleev M.A., Dashkevich N.M., Ataullakhanov F.I. 2015. Haemostasis and thrombosis beyond biochemistry: Roles of geometry, flow and diffusion. Thromb. Res. 136 (4), 699–711.
Dashkevich N.M., Ovanesov M.V., Balandina A.N., Karamzin S.S., Shestakov P.I., Soshitova N.P., Tokarev A.A., Panteleev M.A., Ataullakhanov F.I. 2012. Thrombin activity propagates in space during blood coagulation as an excitation wave. Biophys. J. 103 (10), 2233–2240.
Balandina A.N., Shibeko A.M., Kireev D.A., Novikova A.A., Shmirev I.I., Panteleev M.A., Ataullakhanov F.I. 2011. Positive feedback loops for factor V and factor VII activation supply sensitivity to local surface tissue factor density during blood coagulation. Biophys. J. 101 (8), 1816–1824.
Shibeko A.M., Lobanova E.S., Panteleev M.A., Ataullakhanov F.I. 2010. Blood flow controls coagulation onset via the positive feedback of factor VII activation by factor Xa. BMC Syst. Biol. 4, 5.
Mann K.G., Nesheim M.E., Church W.R., Haley P., Krishnaswamy S. 1990. Surface-dependent reactions of the vitamin K-dependent enzyme complexes. Blood. 76 (1), 1–16.
Podoplelova N.A., Nechipurenko D.Y., Ignatova A.A., Sveshnikova A.N., Panteleev M.A. 2021. Procoagulant platelets: Mechanisms of generation and action. Hamostaseologie. 41 (2), 146–153.
Kovalenko T.A., Panteleev M.A., Sveshnikova A.N. 2017. Substrate delivery mechanism and the role of membrane curvature in factor X activation by extrinsic tenase. J. Theor. Biol. 435, 125–133.
Panteleev M.A., Ananyeva N.M., Greco N.J., Ataullakhanov F.I., Saenko E.L. 2006. Factor VIIIa regulates substrate delivery to the intrinsic factor X-activating complex. FEBS J. 273 (2), 374–387.
Panteleev M.A., Saenko E.L., Ananyeva N.M., Ataullakhanov F.I. 2004. Kinetics of Factor X activation by the membrane-bound complex of Factor IXa and Factor VIIIa. Biochem. J. 381 (Pt 3), 779–794.
Podoplelova N.A., Sveshnikova A.N., Kotova Y.N., Eckly A., Receveur N., Nechipurenko D.Y., Obydennyi S.I., Kireev I.I., Gachet C., Ataullakhanov F.I., Mangin P.H., Panteleev M.A. 2016. Coagulation factors bound to procoagulant platelets concentrate in cap structures to promote clotting. Blood. 128 (13), 1745–1755.
Podoplelova N.A., Sveshnikova A.N., Kurasawa J.H., Sarafanov A.G., Chambost H., Vasil’ev S.A., Demina I.A., Ataullakhanov F.I., Alessi M.C., Panteleev M.A. 2016. Hysteresis-like binding of coagulation factors X/Xa to procoagulant activated platelets and phospholipids results from multistep association and membrane-dependent multimerization. Biochim. Biophys. Acta. 1858 (6), 1216–1227.
Zakharova N.V., Artemenko E.O., Podoplelova N.A., Sveshnikova A.N., Demina I.A., Ataullakhanov F.I., Panteleev M.A. 2015. Platelet surface-associated activation and secretion-mediated inhibition of coagulation factor XII. PLoS One. 10 (2), e0116665.
Terentyeva V.A., Sveshnikova A.N., Panteleev M.A. 2015. Kinetics and mechanisms of surface-dependent coagulation factor XII activation. J. Theor. Biol. 382, 235–243.
Lipets E., Vlasova O., Urnova E., Margolin O., Soloveva A., Ostapushchenko O., Andersen J., Ataullakhanov F., Panteleev M. 2014. Circulating contact-pathway-activating microparticles together with factors IXa and XIa induce spontaneous clotting in plasma of hematology and cardiologic patients. PLoS One. 9 (1), e87692.
Denorme F., Langhauser F., Desender L., Vandenbulcke A., Rottensteiner H., Plaimauer B., Francois O., Andersson T., Deckmyn H., Scheiflinger F., Kleinschnitz C., Vanhoorelbeke K., De Meyer S.F. 2016. ADAMTS13-mediated thrombolysis of t-PA-resistant occlusions in ischemic stroke in mice. Blood. 127 (19), 2337–2345.
Gilbert G.E., Novakovic V.A., Shi J., Rasmussen J., Pipe S.W. 2015. Platelet binding sites for factor VIII in relation to fibrin and phosphatidylserine. Blood. 126 (10), 1237–1244.
Laridan E., Denorme F., Desender L., Francois O., Andersson T., Deckmyn H., Vanhoorelbeke K., De Meyer S.F. 2017. Neutrophil extracellular traps in ischemic stroke thrombi. Ann. Neurol. 82 (2), 223–232.
Morrissey J.H., Choi S.H., Smith S.A. 2012. Polyphosphate: An ancient molecule that links platelets, coagulation, and inflammation. Blood. 119 (25), 5972–5979.
Muller F., Mutch N.J., Schenk W.A., Smith S.A., Esterl L., Spronk H.M., Schmidbauer S., Gahl W.A., Morrissey J.H., Renne T. 2009. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 139 (6), 1143–1156.
Smith S.A., Mutch N.J., Baskar D., Rohloff P., Docampo R., Morrissey J.H. 2006. Polyphosphate modulates blood coagulation and fibrinolysis. Proc. Natl. Acad. Sci. USA. 103 (4), 903–908.
Staessens S., Denorme F., Francois O., Desender L., Dewaele T., Vanacker P., Deckmyn H., Vanhoorelbeke K., Andersson T., De Meyer S.F. 2020. Structural analysis of ischemic stroke thrombi: Histological indications for therapy resistance. Haematologica. 105 (2), 498–507.
Staessens S., Francois O., Desender L., Vanacker P., Dewaele T., Sciot R., Vanhoorelbeke K., Andersson T., De Meyer S.F. 2021. Detailed histological analysis of a thrombectomy-resistant ischemic stroke thrombus: A case report. Thromb. J. 19 (1), 11.
Dohrmann M., Makhoul S., Gross K., Krause M., Pillitteri D., von Auer C., Walter U., Lutz J., Volf I., Kehrel B.E., Jurk K. 2020. CD36-fibrin interaction propagates FXI-dependent thrombin generation of human platelets. FASEB J. 34 (7), 9337–9357.
Nechipurenko D.Y., Receveur N., Yakimenko A.O., Shepelyuk T.O., Yakusheva A.A., Kerimov R.R., Obydennyy S.I., Eckly A., Leon C., Gachet C., Grishchuk E.L., Ataullakhanov F.I., Mangin P.H., Panteleev M.A. 2019. Clot contraction drives the translocation of procoagulant platelets to thrombus surface. Arterioscler. Thromb. Vasc. Biol. 39 (1), 37–47.
Kovalenko T.A., Giraud M.N., Eckly A., Ribba A.S., Proamer F., Fraboulet S., Podoplelova N.A., Valentin J., Panteleev M.A., Gonelle-Gispert C., Cook S., Lafa-nechere L., Sveshnikova A.N., Sadoul K. 2021. Asymmetrical forces dictate the distribution and morphology of platelets in blood clots. Cells. 10 (3), 584.
Zhou G.Q., Zhong W.Z. 1982. Diffusion-controlled reactions of enzymes. A comparison between Chou’s model and Alberty–Hammes–Eigen’s model. Eur. J. Biochem. 128 (2–3), 383–387.
Nesheim M.E., Tracy R.P., Mann K.G. 1984. “Clotspeed,” a mathematical simulation of the functional properties of prothrombinase. J. Biol. Chem. 259 (3), 1447–1453.
Obydennyy S.I., Sveshnikova A.N., Ataullakhanov F.I., Panteleev M.A. 2016. Dynamics of calcium spiking, mitochondrial collapse and phosphatidylserine exposure in platelet subpopulations during activation. J. Thromb. Haemost. 14 (9), 1867–1881.
Panteleev M.A., Ananyeva N.M., Greco N.J., Ataullakhanov F.I., Saenko E.L. 2005. Two subpopulations of thrombin-activated platelets differ in their binding of the components of the intrinsic factor X-activating complex. J. Thromb. Haemost. 3 (11), 2545–2553.
Sveshnikova A.N., Ataullakhanov F.I, Panteleev M.A. 2015. Compartmentalized calcium signaling triggers subpopulation formation upon platelet activation through PAR1. Mol. Biosyst. 11 (4), 1052–1060.
Sveshnikova A.N., Balatskiy A.V., Demianova A.S., Shepelyuk T.O., Shakhidzhanov S.S., Balatskaya M.N., Pichugin A.V., Ataullakhanov F.I., Panteleev M.A. 2016. Systems biology insights into the meaning of the platelet’s dual-receptor thrombin signaling. J. Thromb. Haemost. 14 (10), 2045–2057.
Topalov N.N., Kotova Y.N., Vasil’ev S.A., Panteleev M.A. 2012. Identification of signal transduction pathways involved in the formation of platelet subpopulations upon activation. Br. J. Haematol. 157 (1), 105–115.
Topalov N.N., Yakimenko A.O., Canault M., Artemenko E.O., Zakharova N.V., Abaeva A.A., Loosveld M., Ataullakhanov F.I., Nurden A.T., Alessi M.C., Panteleev M.A. 2012. Two types of procoagulant platelets are formed upon physiological activation and are controlled by integrin alpha(IIb)beta(3). Arterioscler. Thromb. Vasc. Biol. 32 (10), 2475–2483.
Yakimenko A.O., Verholomova F.Y., Kotova Y.N., Ataullakhanov F.I., Panteleev M.A. 2012. Identification of different proaggregatory abilities of activated platelet subpopulations. Biophys. J. 102 (10), 2261–2269.
Majumder R., Weinreb G., Lentz B.R. 2005. Efficient thrombin generation requires molecular phosphatidylserine, not a membrane surface. Biochemistry. 44 (51), 16 998–17 006.
Hoffman M., Monroe D.M., 3rd. 2001. A cell-based model of haemostasis. Thromb. Haemost. 85 (6), 958–965.
Panteleev M.A., Ovanesov M.V., Kireev D.A., Shibeko A.M., Sinauridze E.I., Ananyeva N.M., Butylin A.A., Saenko E.L., Ataullakhanov F.I. 2006. Spatial propagation and localization of blood coagulation are regulated by intrinsic and protein C pathways, respectively. Biophys. J. 90 (5), 1489–1500.
Reitsma S.E., Pang J., Raghunathan V., Shatzel J.J., Lorentz C.U., Tucker E.I., Gruber A., Gailani D., McCarty O.J.T., Puy C. 2021. Role of platelets in regulating activated coagulation factor XI activity. Am. J. Physiol. Cell Physiol. 320 (3), C365–C374.
Hathcock J.J., Nemerson Y. 2004. Platelet deposition inhibits tissue factor activity: In vitro clots are impermeable to factor Xa. Blood. 104 (1), 123–127.
Koklic T., Majumder R., Weinreb G.E., Lentz B.R. 2009. Factor XA binding to phosphatidylserine-containing membranes produces an inactive membrane-bound dimer. Biophys. J. 97 (8), 2232–2241.
Yuan Y., Alwis I., Wu M.C., Kaplan Z., Ashworth K., Bark D., Pham A., Mcfadyen J., Schoenwaelder S.M., Josefsson E.C., Kile B.T. 2017. Neutrophil macroaggregates promote widespread pulmonary thrombosis after gut ischemia. Sci. Trans. Med. 9 (409), eaam5861.
Abaeva A.A., Canault M., Kotova Y.N., Obydennyy S.I., Yakimenko A.O., Podoplelova N.A., Kolyadko V.N., Chambost H., Mazurov A.V., Ataullakhanov F.I., Nurden A.T., Alessi M.C., Panteleev M.A. 2013. Procoagulant platelets form an alpha-granule protein-covered “cap” on their surface that promotes their attachment to aggregates. J. Biol. Chem. 288 (41), 29621–29632.
Kaplan Z.S., Zarpellon A., Alwis I., Yuan Y., McFadyen J., Ghasemzadeh M., Schoenwaelder S.M., Ruggeri Z.M., Jackson S.P. 2015. Thrombin-dependent intravascular leukocyte trafficking regulated by fibrin and the platelet receptors GPIb and PAR4. Nat. Commun. 6, 7835.
Ruggeri Z.M. 2007. The role of von Willebrand factor in thrombus formation. Thromb Res. 120 Suppl 1, S5–9.
Nieswandt B., Watson S.P. 2003. Platelet-collagen interaction: Is GPVI the central receptor? Blood. 102 (2), 449–461.
Qiu Y., Brown A.C., Myers D.R., Sakurai Y., Mannino R.G., Tran R., Ahn B., Hardy E.T., Kee M.F., Kumar S., Bao G., Barker T.H., Lam W.A. 2014. Platelet mechanosensing of substrate stiffness during clot formation mediates adhesion, spreading, and activation. Proc. Natl. Acad. Sci. USA. 111 (40), 14430–14435.
Barnes M.J., MacIntyre D.E. 1979. Platelet-reactivity of isolated constituents of the blood vessel wall. Haemostasis. 8 (3–5), 158–170.
Gutierrez E., Petrich B.G., Shattil S.J., Ginsberg M.H., Groisman A., Kasirer-Friede A. 2008. Microfluidic devices for studies of shear-dependent platelet adhesion. Lab. Chip. 8 (9), 1486–1495.
van Geffen J.P., Brouns S.L.N., Batista J., McKinney H., Kempster C., Nagy M., Sivapalaratnam S., Baaten C., Bourry N., Frontini M., Jurk K., Krause M., Pillitteri D., Swieringa F., Verdoold R., Cavill R., Kuijpers M.J.E., Ouwehand W.H., Downes K., Heemskerk J.W.M. 2019. High-throughput elucidation of thrombus formation reveals sources of platelet function variability. Haematologica. 104 (6), 1256–1267.
Welsh J.D., Poventud-Fuentes I., Sampietro S., Diamond S.L., Stalker T.J., Brass L.F. 2017. Hierarchical organization of the hemostatic response to penetrating injuries in the mouse macrovasculature. J. Thromb. Haemost. 15 (3), 526–537.
van der Meijden P.E., Munnix I.C., Auger J.M., Govers-Riemslag J.W., Cosemans J.M., Kuijpers M.J., Spronk H.M., Watson S.P., Renne T., Heemskerk J.W. 2009. Dual role of collagen in factor XII-dependent thrombus formation. Blood. 114 (4), 881–890.
Faxalv L., Boknas N., Strom J.O., Tengvall P., Theodorsson E., Ramstrom S., Lindahl T.L. 2013. Putting polyphosphates to the test: Evidence against platelet-induced activation of factor XII. Blood. 122 (23), 3818–3824.
Thalin C., Hisada Y., Lundstrom S., Mackman N., Wallen H. 2019. Neutrophil extracellular traps: Villains and targets in arterial, venous, and cancer-associated thrombosis. Arterioscler. Thromb. Vasc. Biol. 39 (9), 1724–1738.
Funding
The work was supported by the Russian Science Foundation (project no. 20-45-01014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare the absence of obvious and potential conflicts of interest related to the publication of this article.
This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by E. Beresneva
Rights and permissions
About this article
Cite this article
Panteleev, M.A., Shibeko, A.M., Nechipurenko, D.Y. et al. Haemostasis and Thrombosis. Spatial Organization of the Biochemical Processes at Microscale. Biochem. Moscow Suppl. Ser. A 16, 107–114 (2022). https://doi.org/10.1134/S1990747822030084
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747822030084