Abstract
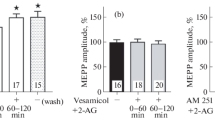
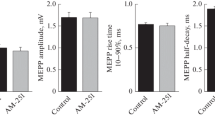
We studied the effects of the endocannabinoids anandamide (AEA) and 2-arachidonoylglycerol (2-AG) on the evoked activity of neuromuscular junctions (NMJs) of mouse diaphragm and m. extensor digitorum longus (m. EDL). Using the microelectrode technique, spontaneous miniature endplate potentials (MEPPs) and multiquantal endplate potentials (EPPs) evoked by short rhythmic nerve stimulation trains (50 Hz, 1 s) were recorded. AEA (30 µM) caused an increase in the MEPP frequency but not in the MEPP amplitude, which lead to an increase in amplitude and quantal content (QC) of each EPP in a train. Quantal analysis showed that AEA causes an enlargement of the size of the readily releasable pool (RRP) of vesicles in motor terminals. The AEA-induced increase in EPP amplitude and QC was prevented by L-type Ca2+-channel blocker nitrendipine (1 µM), which suggests that this channel type is upregulated upon the AEA application. 2-AG (1 µM) caused an increase in MEPP amplitude but not in their frequency in mouse diaphragm NMJs. This was accompanied by an increase in the EPP amplitude, whereas their QC remained at the control level. The same effect was reproduced in synapses of m. EDL. The rise of the EPP amplitude caused by 2-AG was prevented by PKA inhibition by H-89 (1 µM). These data, together with previous evidence that blocking vesicular transport of acetylcholine (ACh) abolishes the 2-AG-induced increase in MEPP amplitude, allows us to suggest that 2-AG stimulates ACh pumping into vesicles in a PKA-dependent way, thus causing an increase in the size of a single ACh quanta. This work is the first to show a fast noncanonical facilitating action of AEA and 2-AG on the evoked activity of mouse NMJ. AEA and 2-AG cause similar presynaptic and CB1-receptor-dependent changes of neuromuscular transmission, but they exert their effects through two different ways, leading to facilitation of different parameters of ACh secretion.





Similar content being viewed by others
REFERENCES
Nicolussi S., Gertsch J. 2015. Endocannabinoid transport revisited. Vitam. Horm. 98, 441–485. https://doi.org/10.1016/bs.vh.2014.12.011
Silver R.J. 2019. The endocannabinoid system of animals. Animals. 9, 686. https://doi.org/10.3390/ani9090686
Zou S., Kumar U. 2018. Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. Int. J. Mol. Sci. 19, 833. https://doi.org/10.3390/ijms19030833
Cristino L., Bisogno T., Di Marzo V. 2020. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 16, 9–29. https://doi.org/10.1038/s41582-019-0284-z
Sugiura T., Kondo S., Sukagawa A., Nakane S., Shinoda A., Itoh K., Yamashita A., Waku K. 1995. 2-arachidonoylglycerol: A possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 215, 89–97. https://doi.org/10.1006/bbrc.1995.2437
Chevaleyre V., Takahashi K.A., Castillo P.E. 2006. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu. Rev. Neurosci. 29, 37–76. https://doi.org/10.1146/annurev.neuro.29.051605.112834
Kreitzer A.C., Regehr W.G. 2002. Retrograde signaling by endocannabinoids. Curr. Opin. Neurobiol. 12, 324–330. https://doi.org/10.1016/S0959-4388(02)00328-8
Lovinger D.M. 2008. Presynaptic modulation by endocannabinoids. Handb. Exp. Pharmacol. 184, 435–477. https://doi.org/10.1007/978-3-540-74805-2_14
Kano M., Ohno-Shosaku T., Hashimotodani Y., Uchigashima M., Watanabe M. 2009. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 89, 309–380.https://doi.org/10.1152/physrev.00019.2008
Castillo P.E., Younts T.J., Chávez A.E., Hashimotodani Y. 2012. Endocannabinoid signaling and synaptic function. Neuron. 76, 70–81. https://doi.org/10.1016/j.neuron.2012.09.020
Cavuoto P., McAinch A.J., Hatzinikolas G., Janovská A., Game P., Wittert G.A. 2007. The expression of receptors for endocannabinoids in human and rodent skeletal muscle. Biochem. Biophys. Res. Commun. 364, 105–110. https://doi.org/10.1016/j.bbrc.2007.09.099
Crespillo A., Suárez J., Bermúdez-Silva F.J., Rivera P., Vida M., Alonso M., Palomino A., Lucena M., Serrano A., Pérez-Martín M., Macias M., Fernández-Llébrez P., Rodríguez de Fonseca F. 2011. Expression of the cannabinoid system in muscle: Effects of a high-fat diet and CB1 receptor blockade. Biochem. J. 433, 175–185. https://doi.org/10.1042/BJ20100751
Hutchins-Wiese H.L., Li Y., Hannon K., Watkins B.A. 2012. Hind limb suspension and long-chain omega-3 PUFA increase mRNA endocannabinoid system levels in skeletal muscle. J. Nutr. Biochem. 23, 986–993. https://doi.org/10.1016/j.jnutbio.2011.05.005
Maccarrone M., Bab I., Bíró T., Cabral G.A., Dey S.K., Di Marzo V., Konje J.C., Kunos G., Mechoulam R., Pacher P., Sharkey K.A., Zimmer A. 2015. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol. Sci. 36, 277–296. https://doi.org/10.1016/j.tips.2015.02.008
Van der Kloot W. 1994. Anandamide, a naturally-occurring agonist of the cannabinoid receptor, blocks adenylate cyclase at the frog neuromuscular junction. Brain Res. 649, 181–184. https://doi.org/10.1016/0006-8993(94)91062-6
Sánchez-Pastor E., Trujillo X., Huerta M., Andrade F. 2007. Effects of cannabinoids on synaptic transmission in the frog neuromuscular junction. J. Pharmacol. Exp. Ther. 321, 439–445. https://doi.org/10.1124/jpet.106.116319
Silveira P.E., Silveira N.A., de Cássia Morini V., Kushmerick C., Naves L.A. 2010. Opposing effects of cannabinoids and vanilloids on evoked quantal release at the frog neuromuscular junction. Neurosci. Lett. 473, 97–101. https://doi.org/10.1016/j.neulet.2010.02.026
Morsch M., Protti D.A., Cheng D., Braet F., Chung R.S., Reddel S.W., Phillips W.D. 2018. Cannabinoid-induced increase of quantal size and enhanced neuromuscular transmission. Sci. Rep. 8, 4685. https://doi.org/10.1038/s41598-018-22888-4
Gaydukov A.E., Dzhalagoniya I.Z., Tarasova E.O., Balezina O.P. 2020. The participation of endocannabinoid receptors in the regulation of spontaneous synaptic activity at neuromuscular junctions of mice. Biochem. (Moscow) Suppl. Series A: Membr. Cell Biol. 14, 7–16. https://doi.org/10.1134/S1990747819060059
Tarasova E.O., Khotkina N.A., Gaydukov A.E., Balezina O.P. 2021. Spontaneous acetylcholine release potentiation induced by 2-arachidonoylglycerol and anandamide in mouse motor synapses. Moscow Univ. Biol. Sci. Bull. 76, 1–6. https://doi.org/10.3103/S0096392521010053
Tarasova E.O., Miteva A.S., Gaidukov A.E., Balezina O.P. 2015. The role of adenosine receptors and L-type calcium channels in the regulation of the mediator secretion in mouse motor synapses. Biochem. (Moscow). Suppl. Series A: Membr. Cell Biol. 9, 318–328. https://doi.org/10.1134/S1990747815050141
Gaydukov A.E., Bogacheva P.O., Balezina O.P. 2019. The participation of presynaptic alpha7 nicotinic acetylcholine receptors in the inhibition of acetylcholine release during long-term activity of mouse motor synapses. Neurochem. J. 13, 20–27. https://doi.org/10.1134/s1819712419010082
McLachlan E.M., Martin A.R. 1981. Non-linear summation of end-plate potentials in the frog and mouse. J. Physiol. 311, 307–324. https://doi.org/10.1113/jphysiol.1981.sp013586
Ruiz R., Cano R., Casañas J.J., Gaffield M.A., Betz W.J., Tabares L. 2011. Active zones and the readily releasable pool of synaptic vesicles at the neuromuscular junction of the mouse. J. Neurosci. 31, 2000–2008. https://doi.org/10.1523/JNEUROSCI.4663-10.2011
Miteva A.S., Gaydukov A.E., Shestopalov V.I., Balezina O.P. 2018. Mechanism of P2X7 receptor-dependent enhancement of neuromuscular transmission in pannexin 1 knockout mice. Purin. Signal. 14, 459–469. https://doi.org/10.1007/s11302-018-9630-7
Gaydukov A.E., Melnikova S.N., Balezina O.P. 2009. Facilitation of acetylcholine secretion in mouse motor synapses caused by calcium release from depots upon activation of L-type calcium channels. Bull. Exp. Biol. Med. 148, 163–166. https://doi.org/10.1007/s10517-009-0678-9
Tarasova E.O., Gaydukov A.E., Balezina O.P. 2015. Methods of activation and the role of calcium/calmodulin-dependent protein kinase II in the regulation of acetylcholine secretion in the motor synapses of mice. Neurochem. J. 9, 101–107. https://doi.org/10.1134/S1819712415020099
Gaydukov A.E., Tarasova E.O., Balezina O.P. 2013. Calcium-dependent phosphatase calcineurin downregulates evoked neurotransmitter release in neuromuscular junctions of mice. Neurochem. J. 7, 29–33. https://doi.org/10.1134/S1819712413010030
Urbano F.J., Depetris R.S., Uchitel O.D. 2001. Coupling of L-type calcium channels to neurotransmitter release at mouse motor nerve terminals. Pflügers Arch. 441, 824–831. https://doi.org/10.1007/s004240000489
Flink M.T., Atchison W.D. 2003. Iberiotoxin-induced block of Ca2+-activated K+ channels induces dihydropyridine sensitivity of ACh release from mammalian motor nerve terminals. J. Pharmacol. Exp. Ther. 305, 646–652. https://doi.org/10.1124/jpet.102.046102
Pagani R., Song M., McEnery M., Qin N., Tsien R.W., Toro L. Stefani E., Uchitel O.D. 2004. Differential expression of alpha 1 and beta subunits of voltage dependent Ca2+ channel at the neuromuscular junction of normal and P/Q Ca2+ channel knockout mouse. Neuroscience, 123, 75–85. https://doi.org/10.1016/j.neuroscience.2003.09.019
Gaydukov A.E., Bogacheva P.O., Balezina O.P. 2016. Calcitonin gene-related peptide increases acetylcholine quantal size in neuromuscular junctions of mice. Neurosci. Lett. 628, 17–23. https://doi.org/10.1016/j.neulet.2016.06.014
Van Der Kloot W., Benjamin W.B., Balezina O.P. 1998. Calcitonin gene-related peptide acts presynaptically to increase quantal size and output at frog neuromuscular junctions. J. Physiol. 507, 689–695. https://doi.org/10.1111/j.1469-7793.1998.689bs.x
Gaydukov A.E, Bogacheva P.O., Tarasova E.O., Molchanova A.I., Miteva A.S., Pravdivceva E.S., Balezina O.P. 2019. Regulation of acetylcholine quantal release by coupled thrombin/BDNF signaling in mouse motor synapses. Cells. 8, 762. https://doi.org/10.3390/cells8070762
Glass M., Felder C.C. 1997. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: Evidence for a G(s) linkage to the CB1 receptor. J. Neurosci. 17, 5327–5333. https://doi.org/10.1523/jneurosci.17-14-05327.1997
Abadji V., Lucas-Lenard J.M., Chin C.N., Kendall D.A. 1999. Involvement of the carboxyl terminus of the third intracellular loop of the cannabinoid CB1 receptor in constitutive activation of G(s). J. Neurochem. 72, 2032–2038. https://doi.org/10.1046/j.1471-4159.1999.0722032.x
Xu J.Y., Zhang J., Chen C. 2012. Long-lasting potentiation of hippocampal synaptic transmission by direct cortical input is mediated via endocannabinoids. J. Physiol. 590, 2305–2315. https://doi.org/10.1113/jphysiol.2011.223511
Cui Y., Prokin I., Xu H., Delord B., Genet S., Venance L., Berry H. 2016. Endocannabinoid dynamics gate spike- timing dependent depression and potentiation. ELife. 5, e13185. https://doi.org/10.7554/eLife.13185
Silva-Cruz A., Carlström M., Ribeiro J.A., Sebastião A.M. 2017. Dual influence of endocannabinoids on long-term potentiation of synaptic transmission. Front. Pharmacol. 8, 921. https://doi.org/10.3389/fphar.2017.00921
Augustin S.M., Lovinger D.M. 2018. Functional relevance of endocannabinoid-dependent synaptic plasticity in the central nervous system ACS Chem. Neurosci. 9, 2146–2161. https://doi.org/10.1021/acschemneuro.7b00508
Piette C., Cui Y., Gervasi N., Venance L. 2020. Lights on endocannabinoid-mediated synaptic potentiation. Front. Mol. Neurosci. 13, 132. https://doi.org/10.3389/fnmol.2020.00132
Varga E., Georgieva T., Tumati S., Alves I., Salamon Z., Tollin G.,Yamamura H.I., Roeske W.R. 2008. Functional selectivity in cannabinoid signaling. Curr. Mol. Pharmacol. 1, 273–284. https://doi.org/10.2174/1874467210801030273
Laprairie R.B., Bagher A.M., Kelly M.E.M., Dupré D.J., Denovan-Wright, E.M. 2014. Type 1 cannabinoid receptor ligands display functional selectivity in a cell culture model of striatal medium spiny projection neurons. J. Biol. Chem. 289, 24845–24862. https://doi.org/10.1074/jbc.M114.557025
Diez-Alarcia R., Ibarra-Lecue I., Lopez-Cardona Á.P., Meana J., Gutierrez-Adán A., Callado L.F., Agirregoitia E., Urigüen L. 2016. Biased agonism of three different cannabinoid receptor agonists in mouse brain cortex. Front. Pharmacol. 7, 415. https://doi.org/10.3389/fphar.2016.00415
Finlay D.B., Cawston E.E., Grimsey N.L., Hunter M.R., Korde A., Vemuri V.K., Makruyannis A., Glass M. 2017. Gαs signalling of the CB1 receptor and the influence of receptor number. Br. J. Pharmacol. 174, 2545–2562. https://doi.org/10.1111/bph.13866
Di Marzo V. 2018. New approaches and challenges to targeting the endocannabinoid system. Nat. Rev. Drug Discov. 17, 623–639. https://doi.org/10.1038/nrd.2018.115
Ibsen M.S., Connor M., Glass M. 2017. Cannabinoid CB1 and CB2 receptor signaling and bias. Cannabis Cannabinoid Res. 2, 48–60. https://doi.org/10.1089/can.2016.0037
Morales P., Bruix M., Jiménez M.A. 2020. Structural insights into β-arrestin/cb1 receptor interaction: Nmr and cd studies on model peptides. Int. J. Mol. Sci. 21, 1–18. https://doi.org/10.3390/ijms21218111
ACKNOWLEDGMENTS
The work was supported by the Russian Foundation for Basic Research (project no. 19-04-00616-a).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
All experimental procedures were performed in accordance with Directive 86/609/EEC on the treatment of humans and laboratory animals and were approved by the Bioethics Commission of the Faculty of Biology of the Moscow State University.
Additional information
Translated by A. Dunina-Barkovskaya
Abbreviations: AEA, anadamide; 2-AG, 2-arachidonoylglycerol; CNS, central nervous system; EPPs, endplate potentials; m. EDL, musculus extensor digitorum longus; MEPPs, miniature endplate potentials; NMJs, neuromuscular junctions; RRP, readily releasable pool.
Rights and permissions
About this article
Cite this article
Tarasova, E.O., Khotkina, N.A., Bogacheva, P.O. et al. Noncanonical Potentiation of Evoked Quantal Release of Acetylcholine by Cannabinoids Anandamide and 2-Arachidonoylglycerol in Mouse Motor Synapses. Biochem. Moscow Suppl. Ser. A 15, 395–405 (2021). https://doi.org/10.1134/S199074782106012X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S199074782106012X