Abstract
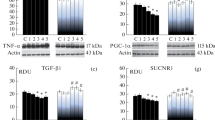
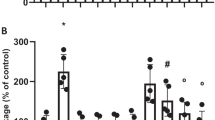
The SUCNR1 succinate receptor (GPR91) is constitutively expressed by the main populations of immunocytes. Succinate, as an immunometabolite (metabokine), performs SUCNR1-dependent modulation of the morphofunctional state of immune cells and is an important regulator of innate and adaptive immunity, as well as tissue homeostasis. Contradictory interpretations of the role of succinate signaling in the functioning of macrophages, the lack of studies of the effect of SUCNR1 on the morphofunctional transformation of microglia, the problem of low permeability of the blood-brain barrier (BBB) for any promising analogues of succinate have predetermined the conduct of this study. The aim of this study was to evaluate the effect of ethylmethylhydroxypyridine succinate on the polarization of microglia in chronic inflammation in the aging brain. The work was performed on white mongrel male rats aged 3 (young), 6 (middle-aged), and 18 months (old). The drug Mexidol (2-ethyl-6-methyl-3-hydroxypyridine succinate) was used as a succinate derivative that overcomes BBB. Mexidol was administered intraperitoneally at a dose of 100 mg/kg daily for 3, 7, and 14 days. Surface markers of the pro- (M1) and anti-inflammatory (M2) phenotype of microglia (CD86 and CD206, respectively), Iba1 cytoplasmic marker of microglia, as well as IL-1β and TNF-α pro-inflammatory cytokines (expressed by M1 microglia), TGF-β1 immunosuppressive cytokine and BDNF neurotrophin (expressed by M2 microglia) were detected in the samples of the cerebral cortex by immunoblotting. It was shown that the expression of cellular markers of pro- (CD86) and especially anti-inflammatory (CD206) microglia decreased in old rats; however, the level of the marker Iba1 that was expressed independently of the phenotype remained unchanged in all the studied groups, which indicated the maintenance of quantitatively equivalent populations of microglia in different age groups. At the same time, the levels of TNF-α and IL-1β increased, and the content of TGF-β1 and BDNF was significantly lower in old rats compared with young and middle-aged ones. In general, the data obtained indicated the dominance of the pro-inflammatory status of microglia in the aging brain. A 14-day course of Mexidol caused a slight decrease in the level of CD86 in old rats and a significant increase of 45% in the content of CD206 up to the level of CD206 in 6-month-old rats. The increase in the CD206 expression was associated with an increase in the levels of TGF-β1 and BDNF by 60% and 35%, respectively, which indicates the involvement of succinate/SUCNR1 signaling in the anti-inflammatory polarization of microglia in the aging brain. The obtained data develop ideas about the cerebral effects of succinate/SUCNR1 signaling and reveal a new component of the mechanism of the neuroprotective action of Mexidol.


Similar content being viewed by others
REFERENCES
He W., Miao F.J.P., Lin D.C.H., Schwandner R.T., Wang Z., Gao J., Chen J.L., Tian H., Ling L. 2004. Citric acid cycle intermediates as ligands for orphan G‑protein-coupled receptors. Nature. 429, 188–193.
Grimolizzi F., Arranz L. 2018. Multiple faces of succinate beyond metabolism in blood. Haematologica. 103 (10), 1586–1592.
Guo Y., Cho S.W., Saxena D., Li X. 2020. Multifaceted actions of succinate as a signaling transmitter vary with its cellular locations. Endocrinol. Metab. 35, 36–43.
Lukyanova L.D., KirovaY.I. 2015. Mitochondria-controlled signalling mechanisms of brain protection in hypoxia. Front. Neurosci. 9, 1–13.
Rubic T., Lametschwandtner G., Jost S., Hinteregger S., Kund J., Carballido-Perrig N., Schwärzler C., Junt T., Voshol H., Meingassner J.G., Mao X., Werner G., Rot A., Carballido J.M. 2008. Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nat. Immunol. 9, 1261–1269.
Caielli S., Veiga D.T., Balasubramanian P., Athale S., Domic B., Murat E., Banchereau R., Xu Z., Chandra M., Chung C.-H., Walters L., Baisch J., Wright T., Punaro M., Lorien N., Stewart K., Fuller J., Ucar D., Ueno H., Zhou J., Banchereau J., Pascual V. 2019. A CD4+ T cell population expanded in lupus blood provides B cell help through interleukin-10 and succinate. Nat. Med. 25 (1), 75–81.
Krzak G., Willis C.M., Smith J.A., Pluchino S., Peruzzotti-Jametti L. 2021. Succinate receptor 1: An emerging regulator of myeloid cell function in inflammation. Trends Immunol. 42 (1), 45–58.
Littlewood-Evans A., Sarret S., Apfel V., Loesle P., Dawson J., Zhang J., Muller A., Tigani B., Kneuer R., Patel S., Valeaux S., Gommermann N., Rubic-Schneider T., Junt T., Carballido J.M. 2016. GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis. J. Exp. Med. 213 (9), 1655–1662.
Diskin C., Palsson-McDermott E.M. 2018. Metabolic modulation in macrophage effector function. Front. Immunol. 9, 270. https://doi.org/10.3389/fimmu.2018.00270
Harber K.J., de Goede K.E., Verberk S.G.S., Meinster E., de Vries H.E., van Weeghel M., de Winther M.P.J., Van den Bossche J. 2020. Succinate is an inflammation-induced immunoregulatory metabolite in macrophages. Metabolites. 10 (9), 372. https://doi.org/10.3390/metabo10090372
Peruzzotti-Jametti L., Bernstock J.D., Vicario N., Costa A.S.H., Kwok C.K., Leonardi T., Booty L.M., Bicci I., Balzarotti B., Volpe G., Mallucci G., Manferrari G., Donega M., Iraci N., Braga A., Hallenbeck J.M., Murphy M.P., Edenhofer F., Frezza C., Pluchino S. 2018. Macrophage-derived extracellular succinate licenses neural stem cells to suppress chronic neuroinflammation. Cell. Stem. Cell. 22 (3), 355–368.
Keiran N., Ceperuelo-Mallafre V., Calvo E., Hernandez-Alvarez M.I., Ejarque M., Nunez-Roa C., Horrillo D., Maymo-Masip E., Rodriguez M.M., Fradera R., de la Rosa J.V., Jorba R., Megia A., Zorzano A., Medina-Gomez G., Serena C., Castrillo A., Vendrell J., Fernandez-Veledo S. 2019. SUCNR1 controls an anti-inflammatory program in macrophages to regulate the metabolic response to obesity. Nat. Immunol. 20 (5), 581–592.
Rodriguez-Gomez J.A., Kavanagh E., Engskog-Vlachos P., Engskog M.K.R., Herrera A.J., Espinosa-Oliva A.M., Joseph B., Hajji N., Venero J.L., Burguillos M.A. 2020. Microglia: Agents of the CNS pro-inflammatory response. Cells. 9 (7), 1717. https://doi.org/10.3390/cells9071717
Voronina T.A. 2009. Mexidol: The main neuropsychotropic effects and mechanism of action. Farmateka (Rus.). 180 (6), 1–4.
Voronina T.A. 2012. Mexidol: The spectrum of pharmacological effects. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova (Rus.). 112 (12), 86–90.
Dumaev K.M., Voronina T.A., Smirnov L.D. 1995. Antioksidanti v profilaktike i terapii patologiy CNS (Antioxidants in the prevention and therapy of CNS pathologies). M.: Institute of Biomed. Chemistry of the Russian Academy of Medical Sciences.
Pozhilova E.V., Novikov V.E., Novikova A.V. 2013. Pharmacodynamics and clinical use of drugs based on hydroxypyridine. Vestnik Smolenskoy Gos. Med. Aademii (Rus.). 12 (3), 56–66.
Shchulkin A.V., Yakusheva E.N., Chernykh I.V. 2014. Distribution of mexidol in the structures of the brain, its cellular elements and subcellular fractions. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova (Rus.). 114 (8), 169–172.
Lukyanova L.D. 2019. Signalnie mehanizmi gipoksii (Signaling mechanisms of hypoxia) M.: RAS.
Shchulkin A.V. 2012. The effect of mexidol on the development of the phenomenon of the neuronal excitotoxicity in vitro. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova (Rus.). 112 (2), 35–39.
Voronina T.A., Kutepova O.A. 2003. Effect of an antioxidant from the class 3-oxypyridine on the functional activity of the central nervous system of mice of different age groups. Zhurnal Vysshey Nervnoy Deiatelnosty Imeni I.P. Pavlova (Rus.). 38 (6), 1126–1131.
Voronina T. A. 2020. Geroprotective effects of ethylmethylhydroxypyridine succinate in an experimental study. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova (Rus.). 120 (4), 81–87.
Kirova Yu.I., Shakova F.M., Germanova E.L., Romanova G.A., Voronina T.A. 2020. The effect of Mexidol on cerebral mitochondriogenesis at a young age and during aging. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova (Rus.). 120 (1), 62–69.
Jiang C.T., Wu W.F., Deng Y.H., Ge J.W. 2020. Modulators of microglia activation and polarization in ischemic stroke. Mol. Med. Reports. 21 (5), 2006–2018.
Baghirova S., Hughes B.G., Hendzel M.J., Schulz R. 2015. Sequential fractionation and isolation of subcellular proteins from tissue or cultured cells. MethodX. 2, 440–445.
Amici S.A., Dong J., Guerau-de-Arellano M. 2017. Molecular mechanisms modulating the phenotype of macrophages and microglia. Front. Immunol. 8, 1520. https://doi.org/10.3389/fimmu.2017.01520
Furman N.T., Gottlieb A., Prabhakara K.S., Bedi S., Caplan Henry W., Ruppert K.A., Srivastava A.K., Olson S.D., Cox Jr. C.S. 2020. High‑resolution and differential analysis of rat microglial markers in traumatic brain injury: Conventional flow cytometric and bioinformatics analysis. Sci. Report. 10, 11991–12005.
Jurga A.M., Paleczna M., Kuter K.Z. 2020. Overview of general and discriminating markers of differential microglia phenotypes. Front. Cell. Neurosci. 14, 198. https://doi.org/10.3389/fncel.2020.00198
Angelova D.M., Brown D.R. 2019. Microglia and the aging brain: Are senescent microglia the key to neurodegeneration? J. Neurochem. 151, 676–688.
Luo Y., Zheng S.G. 2016. Hall of fame among pro-inflammatory cytokines: interleukin-6 gene and its transcriptional regulation mechanisms. Front. Immunol. 7, 604. https://doi.org/10.3389/fimmu.2016.00604
Hamel D., Sanchez M., Duhamel F., Roy O., Honore J.-C., Noueihed B., Zhou T., Nadeau-Vallee M., Hou X., Lavoie J.-C., Mitchell G., Mamer O.A., Chemtob S. 2014. G-protein–coupled receptor 91 and succinate are key contributors in neonatal postcerebral hypoxia-ischemia recovery. Arterioscler. Thromb. Vasc. Biol. 34, 285–293.
Bratic A., Larsson N.-G. 2013. The role of mitochondria in aging. J. Clin. Invest. 123 (3), 951–957.
Gilissen J., Jouret F., Pirotte B., Hanson J. 2016. Insight into SUCNR1 (GPR91) structure and function. Pharmacol. Ther. 159, 56–65.
Greer P.L., Greenberg M.E. 2008. From synapse to nucleus: Calcium-dependent gene transcription in the control of synapse development and function. Neuron. 59 (6), 846–860.
Kitagawa K. 2007. CREB and cAMP response element-mediated gene expression in the ischemic brain. FEBS J. 274, 3210–3217.
Eisele P.S., Salatino S., Sobek J., Hottiger M.O., Handschin C. 2013. The peroxisome proliferator-activated receptor γ coactivator 1α/β (PGC-1) coactivators repress the transcriptional activity of NF-κB in skeletal muscle cells. J. Biol. Chem. 288 (4), 2246–2260.
Torshin I.Yu., Gromova O.A., Sardaryan I.S., Fedotova L.E., Semenov V.A. 2016. Comparative chemoreactome analysis of mexidol. Farmakokinetika i Farmakodinamika (Rus.). 4, 19–30.
ACKNOWLEDGMENTS
The work was carried out within the framework of the research program “Pathogenetic mechanisms of neurodegenerative diseases and the development of their complex therapy” (project no. 0520-2019-0029), planned at the Institute of General Pathology and Pathophysiology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
The experiments involving animals were carried out in accordance with the National Standard of the Russian Fede-ration GOST R-53434-2009 “Principles of good laboratory practice”, Directive 2010/63/EU of the European Parliament and the Council of the European Union on the protection of animals used for scientific purposes. The protocols of the experiments were approved by the Ethics Committee of the FSBSI “Institute of General Pathology and Pathophysiology”.
Additional information
Translated by E. Puchkov
Rights and permissions
About this article
Cite this article
Kirova, Y.I., Shakova, F.M. & Voronina, T.A. Ethylmethylhydroxypyridine Succinate Induces Anti-inflammatory Polarization of Microglia in the Brain of Aging Rat. Biochem. Moscow Suppl. Ser. A 15, 356–364 (2021). https://doi.org/10.1134/S1990747821060040
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747821060040