Abstract
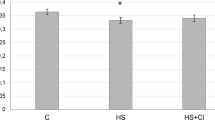
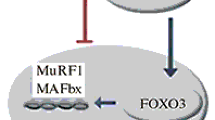
The role of histone deacetylases 4/5 (HDAC4/5) in the activation of myogenin and E3 ligases (MuRF1 and MAFbx) under functional unloading of m. soleus was studied. For this purpose, trichostatin A, a histone deacetylase inhibitor, was used in suspended rats. Wistar rats (24 three-month-old males) were divided into three groups (eight in each). One group served as a control (C), the second (HS) and third groups were suspended for 3 days (the rats of group 3 were intraperitoneally administered trichostatin A at a dose of 0.6 mg/kg of body mass per day (HST)). The rats were killed with an overdose of nembutal (75 mg/kg of mass), and m. soleus was immediately frozen in liquid nitrogen. In the HST group, a significant decrease in the HDAC4 protein content in the nuclear fraction (in contrast to the HS group) and its increase in the cytoplasmic fraction (relative to the control (p < 0.05)) were found. The administration of trichostatin A prevented changes in the HDAC4 content in the nucleus (relative to the C group), whereas the HDAC5 content was significantly reduced (p < 0.05) in the HS group. Thus, the drug prevented the effect of functional unloading on the nuclear localization of HDAC 4/5. The level of myogenin transcription factor in the HST group of rats did not differ from that in the control, while it was significantly higher in the HS group (p < 0.05). The expression of mRNA of E3 ligase atrogin-1/MAFbx in the HST rats was the same as in the control group and was significantly higher in the HS group (p < 0.05). The expression of mRNA of E3 ligase MuRF1in both groups was increased compared to the controls (regardless of the drug administration). Conclusion: HDAC4/5 regulates the expression of myogenin and E3 ligase atrogin-1/MAFbx. Inhibition of HDAC4/5 does not affect the regulation of the expression of E3 ligase MuRF1.




Similar content being viewed by others
REFERENCES
Glass D.J. 2001. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science.294 (5547), 1704–1708.
Brocca L., Toniolo L., Reggiani C., Bottinelli R., Sandri M., Pellegrino M.A. 2017. FoxO-dependent atrogenes vary among catabolic conditions and play a key role in muscle atrophy induced by hindlimb suspension. J. Physiol.595 (4), 1143–1158
Khalil R. 2018. Ubiquitin-proteasome pathway and muscle atrophy. Adv. Exp. Med. Biol. 1088, 235–248.
Belova S.P., Shenkman B.S., Kostrominova T.Y., Nemirovskaya T.L. 2017. Paradoxical effect of IKKβ inhibition on the expression of E3 ubiquitin ligases and unloading-induced skeletal muscle atrophy. Physiol. Rep.5 (16), e13291
Lomonosova Y.N., Shenkman B.S., Nemirovskaya T.L. 2012. Attenuation of unloading-induced rat soleus atrophy with the heat-shock protein inducer 17-(allylamino)-17-demethoxygeldanamycin. FASEB J.26 (10), 4295–4301.
Senf S.M., Sandesara P.B., Reed S.A., Judge A.R. 2011. p300 Acetyltransferase activity differentially regulates the localization and activity of the FOXO homologues in skeletal muscle. Am. J. Physiol. Cell. Physiol. 300, C1490–1501.
Du Bois P., Pablo Tortola C., Lodka D., Kny M., Schmidt F., Song K., Schmidt S., Bassel-Duby R., Olson E.N., Fielitz J. 2015. Angiotensin II induces skeletal muscle atrophy by activating TFEB-mediated MuRF1 expression. Circ Res. 117 (5), 424–436.
Walsha M.E.,Van Remmen H. 2016. Emerging roles for histone deacetylases in age-related muscle atrophy. Nutrition Healthy Aging. 4 (1), 17–30
Dupré-Aucouturier S., Castells J., Freyssenet D., Desplanches D. 2015. Trichostatin A, a histone deacetylase inhibitor, modulates unloaded-induced skeletal muscle atrophy. J. Appl. Physiol.119 (4), 342–351.
Novikov V.E., Ilyin E.A. 1981. Age-related reactions of rat bones to their unloading. Aviat. Space Environ. Med.52 (9), 551–553.
Morey-Holton E., Globus R. 2002. Hindlimb unloading rodent model: Technical aspects. J. Appl. Physiol.92, 1367–1377
Kachaeva E.V., Shenkman B.S. 2012. Various jobs of proteolytic enzymes in skeletal muscle during unloading: Facts and speculations. J. Biomed. Biotechnol.2012, 1–15.
Bodine S.C., Latres E., Baumhueter S., Lai V.K., Nunez L., Clarke B.A., Poueymirou W.T., Panaro F.J., Na E., Dharmarajan K., Pan Z.Q., Valenzuela D.M., DeChiara T.M., Stitt T.N., Yancopoulos G.D., Glass D.J. 2001. Identification of ubiquitin ligases required for skeletal muscle atrophy. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science.294 (5547), 1704–1708.
Bertaggia E., Coletto L., Sandri M. 2012. Posttranslational modifications control FoxO3 activity during denervation. Am. J. Physiol. Cell. Physiol.302, 587–596.
Marek L., Hamacher A., Hansen F.K., Kuna K., Gohlke H., Kassack M.U., Kurz T. 2013. Histone deacetylase (HDAC) inhibitors with a novel connecting unit linker region reveal a selectivity profile for HDAC4 and HDAC5 with improved activity against chemoresistant cancer cells. J. Med. Chem.56 (2), 427–436.
Yoshihara T., Machida S., Kurosaka Y., Kakigi R., Sugiura T., Naito H. 2016. Immobilization induces nuclear accumulation of HDAC4 in rat skeletal muscle. J. Physiol. Sci.66 (4), 337–343.
Kirsh O., Seeler J.S., Pichler A., Gast A., Muller S., Miska E., Mathieu M., Harel-Bellan A, Kouzarides T., Melchior F., Dejean A. 2002. The SUMO E3 ligase RanBP2 promotes modification of the HDAC4 deacetylase. EMBO J.21 (11), 2682–2691.
Kuo M.H., Allis C.D. 1998. Roles of histone acetyltransferases and deacetylases in gene regulation. BioEssays. 20, 615–626.
Miska E.A., Karlsson C., Langley E., Nielsen S.J., Pines J., Kouzarides T. 1999. HDAC4 deacetylase associates with and represses the MEF2 transcription factor. EMBO J.18, 5099–5107.
McKinsey T.A., Zhang C.L., Lu J., Olson E.N. 2000. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature. 408, 106–111.
Moresi V., Williams A.H., Meadows E., Flynn J.M., Potthoff M.J., McAnally J., Shelton J.M., Backs J., Klein W.H., Richardson J.A., Bassel-Duby R., Olson E.N. 2010. Myogenin and class II HDACs control neurogenic muscle atrophy by inducing E3 ubiquitin ligases. Cell. 143 (1), 35–45.
Bricceno K.V., Sampognaro P.J., Van Meerbeke J.P., Sumner C.J., Fischbeck K.H., Burnett B.G. 2012. Histone deacetylase inhibition suppresses myogenin-dependent atrogene activation in spinal muscular atrophy mice. Hum. Mol. Genet.21 (20), 4448–4459.
Furlow J.D., Watson M.L., Waddell D.S., Neff E.S., Baehr L.M., Ross A.P., Bodine S.C. 2013. Altered gene expression patterns in muscle ring finger 1 null mice during denervation- and dexamethasone-induced muscle atrophy. Physiol. Genomics.45 (23), 1168–1185.
Sandri M., Sandri C., Gilbert A., Skurk C., Calabria E., Picard A., Walsh K., Schiaffino S., Lecker S.H., Goldberg A.L. 2004. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell.117, 399–412.
Clavel S., Siffroi-Fernandez S., Coldefy A.S., Boulukos K., Pisani D.F., Dérijard B. 2010. Regulation of the intracellular localization of Foxo3a by stress-activated protein kinase signaling pathways in skeletal muscle cells. Mol. Cell Biol. 30 (2), 470–480.
Bodine S.C., Baehr L.M. 2014. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 307 (6), 469–484.
Mihaylova M.M., Vasquez D.S., Ravnskjaer K., Denechaud P.D., Yu R.T., Alvarez J.G., Downes M., Evans R.M., Montminy M., Shaw R.J. 2011. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell.145, 607–621.
Kuo T., Lew M.J., Mayba O., Harris C.A., Speed T.P., Wang J.C. 2012. Genomewide analysis of glucocorticoid receptor-binding sites in myotubes identifies gene networks modulating insulin signaling. Proc. Natl. Acad. Sci. USA.109, 11 160–11 165.
Greer E.L., Oskoui P.R., Banko M.R., Maniar J.M., Gygi M.P., Gygi S.P., Brunet A. 2007. The energy sensor amp-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J. Biol. Chem.282, 30 107–30 119.
ACKNOWLEDGMENTS
The work was supported by the Russian Science Foundation, project no. 18-15-00062.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors state that there is no conflict of interest.
All procedures were performed in accordance with the European Communities Council Directive (November 24, 1986; 86/609/EEC) and the Declaration on humane treatment of animals. The protocol of experiments was approved by the Bioethics Committee of the Institute of Biomedical Problems, Russian Academy of Sciences.
Additional information
Translated by E. Puchkov
Rights and permissions
About this article
Cite this article
Belova, S.P., Mochalova, E.P. & Nemirovskaya, T.L. The Role of Class IIa HDACs in the Expression of E3 Ligases ATROGIN-1/MAFbx and MuRF1 under Muscle Unloading. Biochem. Moscow Suppl. Ser. A 14, 74–80 (2020). https://doi.org/10.1134/S1990747820010031
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747820010031