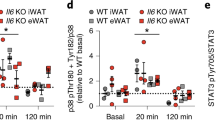
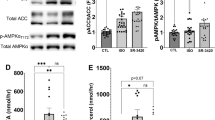
Abstract
Adipocytes of white adipose tissue are the cells maintaining glucose homeostasis in an organism, which is controlled by insulin. Insulin stimulates the translocation of glucose transporter GLUT4 from the cytosol into the cell membrane, as well as glucose transport and utilization in these cells. Here we show that insulin-induced [Ca2+]i oscillations are supported by the two signaling pathways involving: (1) phosphoinositide 3-kinase (PI3K), protein kinase B (Akt/PKB), endothelial NO synthase (eNOS), nitric oxide (NO), and ryanodine receptor (RyR) and (2) phospholipase C (PLC) and inositol 3-phosphate receptor (IP3R). Thus, the PI3K Akt/PKB signaling pathway initiates not only metabolic but also Ca2+-signaling pathways in response to insulin.
Similar content being viewed by others
References
White M.F. 1997. The insulin signalling system and the IRS proteins. Diabetologia. 40, 2–17.
Sun X.J., Rothenberg P., Kahn CR., Backer J.M., Araki E., Wilden P.A., Cahill D.A., Goldstein B.J., White M.F. 1991. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 352, 73–77.
Giorgetti S., Ballotti R., Kowalski-Chauvel A., Cormont M., van Obberghen E. 1992. Insulin stimulates phosphatidylinositol-3-kinase activity in rat adipocytes. Eur. J. Biochem. 207 (2), 599–606.
Vanhaesebroeck B., Alessi D.R. 2000. The PI3KPDK1 connection: More than just a road to PKB. J. Biochem. 346, 561–576.
Cohen P., Alessi D.R., Cross D.A. 1997. PDK1, one of the missing links in insulin signal transduction? FEBS Lett. 410, 3–10.
Hajduch E., Litherland G.J., Hundal HS. 2001. Protein kinase B (PKB/Akt)–a key regulator of glucose transport? FEBS Lett. 492, 199–203.
Dolgacheva L.P., Turovsky E.A., Turovskaya M.V., Zinchenko V.P., Dynnik V.V. 2012. a-Adrenergic control of two Ca2+-signaling pathways of adipocytes. Ross. Fiziol. Zh. im. I.M. Sechenova (Rus.). 98 (12), 47–54.
Turovsky E.A., Turovskaya M.V., Dolgacheva L.P., Zinchenko V.P., Dynnik V.V. 2013. Acetylcholine promotes Ca2+ and NO-oscillations in adipocytes implicating Ca2+ NO cGMP cADP-ribose Ca2+ positive feedback loop–modulatory effects of norepinephrine and atrial natriuretic peptide. PLoS One. 8 (5), 1–18.
Turovsky E.A., Turovskaya M.V., Tolmacheva A.V., Dolgacheva L.P., Zinchenko V.P., Dynnik V.V. 2013. Resistance of mouse adipocytes to noradrenalin in obesity and type 2 diabetes mellitus. Fundamental’nye issledovaniya (Rus.). 1 (3), 595–599.
Trevellin E., Scorzeto M., Olivieri M., Granzotto M., Valerio A., Tedesco L., Fabris R., Serra R., Quarta M., Reggiani C., Nisoli E., Vettor R. 2014. Exercise training induces mitochondrial biogenesis and glucose uptake in subcutaneous adipose tissue through eNOS-dependent mechanisms. Diabetes. 63 (8), 2800–2811.
Nagano T., Yoshimura T. 2002. Bioimaging of nitric oxide. Chem. Rev. 102 (4), 1235–1270.
Fulton D., Gratton J.P., McCabe T.J., Fontana J., Fujio Y., Walsh K., Franke T.F., Papapetropoulos A., Sessa W.C. 1999. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 399 (6736), 597–601.
Dimmeler S., Fleming I., Fisslthaler B., Hermann C., Busse R., Zeiher A.M. 1999. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 399 (6736), 601–605.
Prakash Y.S., Kannan M.S., Walseth T.F., Sieck G.C. 1998. Role of cyclic ADP-ribose in the regulation of [Ca2+]i in porcine tracheal smooth muscle. Am. J. Physiol. 274 (6), 1653–1660.
Jung U.J., Choi M.S. 2014. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 15 (4), 6184–223.
Lillioja S., Mott D.M., Spraul M., Ferraro R., Foley J.E., Ravussin E., Knowler W.C., Bennett P.H., Bogardus C. 1993. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 329, 1988–1992.
Cheatham B., Vlahos C., Cheatham L., Wang L., Blenis J., Kahn C. 1994. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol. Cell. Biol. 14, 4902–4911.
Okada T., Kawano Y., Sakakibara T., Hazeki O., Ui M. 1994. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. Studies with a selective inhibitor wortmannin. J. Biol. Chem. 269, 3568–3573.
Alessi D.R., Downes C.P. 1998. The role of PI 3-kinase in insulin action. Biochim. Biophys. Acta. 1436, 151–164.
Whiteman E.L., Cho H., Birnbaum M.J. 2002. Role of Akt/protein kinase B in metabolism. Trends Endocrinol. Metab. 13, 444–451.
Gonzalez E., McGraw T.E. 2009. The Akt kinases: Isoform specificity in metabolism and cancer. Cell Cycle. 8 (16), 2502–2508.
Hanada M., Feng J., Hemmings B.A. 2004. Structure, regulation and function of PKB/Akt–a major therapeutic target. Biochim. Biophys. Acta. 1697, 3–16.
Manning B.D., Cantley L.C. 2007. Akt/PKB signaling: Navigating downstream. Cell. 129, 1261–1274.
Pfeffer S., Aivazian D. 2004. Targeting Rab GTPases to distinct membrane compartments. Nat. Rev. Mol. Cell. Biol. 5, 886–896.
Stenmark H. 2009. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell. Biol. 10, 513–525.
Zerial M., McBride H. 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell. Biol. 2, 107–117.
Kane S., Sano H., Liu S.C., Asara J.M., Lane W.S., Garner C.C., Lienhard G.E. 2002. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J. Biol. Chem. 277, 22115–22118.
Tan S.X., Ng Y., Burchfield J.G., Ramm G., Lambright D.G., Stockli J., James D.E. 2012. The Rab GTPase-activating protein TBC1D4/AS160 contains an atypical phosphotyrosine-binding domain that interacts with plasma membrane phospholipids to facilitate GLUT4 trafficking in adipocytes. Mol. Cell. Biol. 32 (24), 4946–4959.
Yu H., Rathore S.S., Davis E.M., Ouyang Y., Shen J. 2013. Doc2b promotes GLUT4 exocytosis by activating the SNARE-mediated fusion reaction in a calciumand membrane bending-dependent manner. Mol. Biol. Cell. 24 (8), 1176–1184.
Roy D., Perreault M., Marette A. 1998. Insulin stimulation of glucose uptake in skeletal muscles and adipose tissues in vivo is NO dependent. Am. J. Physiol. 274 (4 Pt 1), 692–699.
Hong Y.H., Betik A.C., McConell G.K. 2014. Role of nitric oxide in skeletal muscle glucose uptake during exercise. Exp. Physiol. 99 (12), 1569–1573.
Masters B.S.S., McMillan K., Sheta E.A., Nishimura J.S., Roman L.J., Martasek P. 1996. Neuronal nitric oxide synthase, a modular enzyme formed by convergent evolution: Structure studies of a cysteine thiolate-liganded heme protein that hydroxylates L-arginine to produce NO as a cellular signal. FASEB J. 10, 552–558.
Fleming I. 2010. Molecular mechanisms underlying the activation of eNOS. Pflügers Archiv. 459, 793–806.
Forstermann U., Sessa W.C. 2012. Nitric oxide synthases: Regulation and function. Eur. Heart J. 33, 829–837.
Venema R.C., Sayegh H.S., Kent J.D., Harrison D.G. 1996. Identification, characterization, and comparison of the calmodulin-binding domains of the endothelial and inducible nitric oxide synthases. J. Biol. Chem. 271, 6435–6440.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.A. Turovsky, M.V. Turovskaya, V.P. Zinchenko, V.V. Dynnik, L.P. Dolgacheva, 2015, published in Biologicheskie Membrany, 2015, Vol. 32, No. 5–6, pp. 429–436.
Rights and permissions
About this article
Cite this article
Turovsky, E.A., Turovskaya, M.V., Zinchenko, V.P. et al. Insulin induces Ca2+ oscillations in white fat adipocytes via PI3K and PLC. Biochem. Moscow Suppl. Ser. A 10, 53–59 (2016). https://doi.org/10.1134/S1990747815050189
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747815050189