Abstract
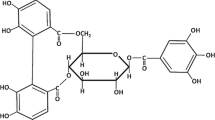
Effects of hydrolysable tannins on plasma membranes of Chara corallina giant cells and lipid bilayer membrane (BLM) were studied. Tannin inhibited chloride channels of alga cell membranes in a dose-dependent manner. Current jumps emerging in BLM in the presence of tannin suggest formation of ion channels, predominantly anion selective. The presence of cholesterol in BLM increased the open state lifetime of the channels. The findings indicate that hydrolysable tannins possess a membrane activity and are capable to form anion channels in a cell membrane.
Similar content being viewed by others
References
Piao X., Piao X.L., Kim H.Y., Cho E.J. 2008. Antioxidative activity of geranium (Pelargonium inquinans Ait) and its active component, 1,2,3,4,6-penta-O-galloyl-β-D-glucose. Phytother. Res. 22(4), 534–538.
Williams R.J., Spencer J.P., Rice-Evans C. 2004, Flavonoids: Antioxidants or signaling molecules? Free Radic. Biol. Med. 36, 838–849
Arora A., Nair M.G., Straasburg G.M. 1998. Antioxidant activities of isoflavones and their biological metabolites in a liposomal system. Arch. Biochem. Biophys. 356, 133–141.
van Acker S.A., van den Berg D.J., Tromp M.N., Griffioen D.H., van Bennekom W.P., van der Vijgh W.J., Bast A. 1996. Structural aspects of antioxidant activity of flavonoids. Free Radic. Biol. Med. 20, 331–342.
Liao K., Yin M. 2000. Individual and combined antioxidant effects of seven phenolic agents in human erythrocyte membrane ghosts and phosphatidylcholine liposome systems: Importance of thepartition coefficient. J. Agric. Food Chem. 48, 2266–2270.
Madsen H.L., Andersen C.M., Jorgensen L.V., Skibsted L.H. 2000. Radical scavenging by dietary flavonoids. A kinetic study of antioxidant efficiencies. Eur. Food Res. Technol. 211, 240–246.
Olchowik E., Sciepuk A., Mavlyanov S., Abdullajanova N., Zamaraeva M. 2012. Antioxidant capacities of polyphenols from Sumac (Rhus typhina L.) leaves in protection of erythrocytes against oxidative damage. Biomedicine and Preventive Nutrition. 2(2), 99–105.
Salikhov Sh., Mavlyanov S.M., Abdulldjanova N.G., Pirniyazov A.Y., Dalimov D.N., Salakhutdinov B.A., Kurmukov A.G. 2006. Polyphenols of some tannin containing plants and creation on their base drug remedies. New Research on Biotechnology and Medicine-US. Ch XI. 109–117.
Islambekov Sh.Yu., Mavlyanov S.M., Kamaev F.G., Ismailov A.I. 1994. Phenolic compounds of sumac. Chem. Nat. Comp. 30, 37–39.
Kuo P.T., Lin T.P., Liu L.C., Huang C.H., Lin J.K., Kao J.Y., Way T.D. 2009. Penta-O-galloyl-β-D-glucose suppresses prostate cancer bone metastasis by transcriptionally repressing EGF-induced MMP-9 expression. J. Agric. Food Chem. 57(8), 3331–3339.
Hu H., Lee H.J., Jiang C., Zhang J., Wang L., Zhao Y., Xiang Q., Lee E.O., Kim S.H., Lu J. 2008. Penta-1,2,3,4,6-O-galloyl-β-D-glucose induces p53 and inhibits STAT3 in prostate cancer cells in vitro and suppresses prostate xenograft tumor growth in vivo. Mol. Cancer Ther. 7(9), 2681–2691.
Peng N., Clark J.T., Prasain J., Kim H., White C.R., Wyss J.M. 2005. Antihypertensive and cognitive effects of grape polyphenols in estrogen-depleted, female, spontaneously hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R771–R775. doi:10.1152/ajpregu.00147.
Huh J.E., Lee E.O., Kim M.S., Kang K.S., Kim C.H., Cha B.C., Surh Y.J., Kim S.H. 2005. Penta-O-galloyl-β-D-glucose suppresses tumor growth via inhibition of angiogenesis and stimulation of apoptosis: Roles of cyclooxygenase-2 and mitogen-activated protein kinase pathways. Carcinogenesis. 26(8), 1436–1445.
Ho L.L., Chen W.J., Lin-Shiau S.Y., Lin J.K. 2002. Penta-O-galloyl-β-D-glucose inhibits the invasion of mouse melanoma by suppressing metalloproteinase-9 through down-regulation of activator protein-1. Eur. J. Pharmacol. 453, 149–158.
Oh G.S., Pae H.O., Oh H., Hong S.G., Kim I.K., Chai K.Y., Yun Y.G., Kwon T.O., Chung H.T. 2001. In vitro anti-proliferative effect of 1,2,3,4,6-penta-O-galloyl-β-D-glucose on human hepatocellular carcinoma cell line, SK-HEP-1 cells. Cancer Lett. 174(1), 17–24.
Chen W.J., Lin J.K. 2004. Induction of G1 arrest and apoptosis in human jurkat T cells by pentagalloyl glucose through inhibiting proteasome activity and elevating p27Kip1, p21Cip1/WAF1, and Bax proteins. J. Biol. Chem. 279(14), 13496–13505.
Hua K.T., Way T.D., Lin J.K. 2006. Pentagalloylglucose inhibits estrogen receptor α by lysosome-dependent depletion and modulates ErbB/PI3K/Akt pathway in human breast cancer MCF-7 cells. Mol. Carcinog. 45(8), 551–560.
Ray D., Sharatchandra Kh., Thokchom I.S., 2006. Antipyretic, antidiarrhoeal, hypoglycaemic and hepatoprotective activities of ethyl acetate extract of Acacia catechu Willd. in albino rats. Indian J. Pharmacol. 38(6), 408–413.
Yu X., Chu S., Hagerman A.E., Lorigan G.A. 2011. Probing the interaction of polyphenols with lipid bilayers by solid-state NMR spectroscopy. J. Agric. Food Chem. 59(12), 6783–6789.
Arora T.M., Byrem M.G., Nair G.M. 2000. Strasburg. Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch. Biochem. Biophys. 373(1), 102–109.
Hendrich A.B. 2006. Flavonoid-membrane interactions: possible consequences for biological effects of some polyphenolic compounds. Acta Pharmacol. Sin. 27(1), 27–40.
Oteiza P.I., Erlejman A.G., Verstraeten S.V., Keen C.L., Fraga C.G. 2005. Flavonoid-membrane interactions: A protective role of flavonoids at the membrane surface? Clin. Dev. Immunol. 12(1), 19–25.
Simon S.A., Disalvo E.A., Gawrisch K., Borovyagin V., Toone E., Schiffman S.S., Needham D., McIntosh T.J. 1994. Increased adhesion between neutral lipid bilayers: interbilayer bridges formed by tannic acid. Biophys. J. 66(6), 1943–1958.
Huh N.W., Porter N.A., McIntosh T.J., Simon S.A. 1996. The interaction of polyphenols with bilayers: Conditions for increasing bilayer adhesion. Biophys. J. 71(6), 3261–3277.
Hadi S.M., Bhat S.H., Azmi A.S., Hanif S., Shamim U., Ullah M.F. 2007. Oxidative breakage of cellular DNA by plant polyphenols: A putative mechanism for anticancer properties. Semin. Cancer Biol. 17(5), 370–376.
Li Y., Kim J., Li J., Liu F., Liu X., Himmeldirk K., Ren Y., Wagner T.E., Chen X. 2005. Natural anti-diabetic compound 1,2,3,4,6-penta-O-galloyl-D-glucopyranose binds to insulin receptor and activates insulin-mediated glucose transport signaling pathway. Biochem. Biophys. Res. Commun. 336(2), 430–437.
Woll K.H., Leibowitz M.D., Neumcke B., Hille B. 1987. A high-conductance anion channel in adult amphibian skeletal muscle. Pflugers Arch. 410(6), 632–640.
Namkung W., Thiagarajah J.R., Phuan P.W., Verkman A.S. 2010. Inhibition of Ca2+-activated Cl− channels by gallotannins as a possible molecular basis for health benefits of red wine and green tea. FASEB J. 24(11), 4178–4186.
Lunevsky V.Z., Zherelova O.M., Vostrikov I.Y., Berestovsky G.N. 1983. Exitation of Characeae cell membranes as result of activation of calcium and chloride channels. J. Membr. Biol. 72, 43–58.
Zherelova O.M., Kataev A.A., Grishchenko V.M., Knyazeva E.L., Permyakov S.E., Permyakov E.A. 2009. Interaction of antitumor alpha-lactalbumin-oleic acid complexes with artificial and natural membranes. J. Bioenerg. Biomembr. 41, 229–237.
Berestovsky G.N., Kataev A.A. 2005. Voltage-gated calcium and Ca2+-activated chloride channels and Ca2+ transients: Voltage-clamp studies of perfused and intact cells of Chara. Eur. Biophys. J. 34, 973–986.
Mueller P., Rudin D.O., Tien H.T. Wescott W.C. 1963. Methods for the formation of single bimolecular lipid membranes in aqueous solution. J. Phys. Chem. 67(2) 534–535.
Olchowiki E., Lotkowski K., Mavlyanov S., Abdullajanova N., Ionov M., Bryszewska M., Zamaraeva M. 2012. Stabilization of erythrocytes against oxidative and hypotonic stress by tannins isolated from sumac leaves (Rhus typhina L.) and grape seeds (Vitis vinifera L.). Cell. Mol. Biol. Lett. 17(3), 333–348.
Beretta G., Artali R., Caneva E., Facino R.M. 2011. Conformation of the tridimensional structure of 1,2,3,4,6-pentagalloyl-β-D-glucopyranose (PGG) by (1)H NMR, NOESY and theoretical study and membrane interaction in a simulated phospholipid bilayer: A first insight. Magn. Reson. Chem. 49(3), 132–136.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © M.P. Borisova, A.A. Kataev, S.M. Mavlyanov, N.G. Abdullajanova, 2014, published in Biologicheskie Membrany, 2014, Vol. 31, No. 4, pp. 278–287.
Rights and permissions
About this article
Cite this article
Borisova, M.P., Kataev, A.A., Mavlyanov, S.M. et al. Effects of hydrolysable tannins on native and artificial biological membranes. Biochem. Moscow Suppl. Ser. A 9, 53–60 (2015). https://doi.org/10.1134/S1990747814040023
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747814040023