Abstract
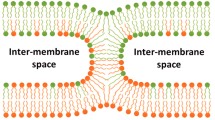
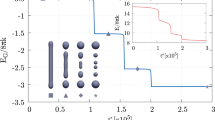
We consider the process of fusion of lipid membranes from the stage of stalk with minimal radius to the stage of fusion pore. We assume that stalk directly developed into the fusion pore, omitting the stage of hemifusion diaphragm. Energy of intermediate stages is calculated on the basis of the classical elasticity theory of liquid crystals adapted for lipid membranes. The trajectory of transition from stalk to pore is obtained with regard to hydrophobic and hydration interactions. Continuous change of orientation of lipids in distal monolayers occurs along the trajectory. The orientation changes from the direction along rotational axis of the system specific to stalk to the direction corresponding to the fusion pore. Dependence of energy of intermediate stages on the value of spontaneous curvature of distal monolayers of the fusing membranes is obtained. We demonstrate that the energy barrier of the stalk-to-pore transition decreases when distal monolayers have positive spontaneous curvature, which is in accordance with available experimental data.
Similar content being viewed by others
References
Chernomordik L.V., Frolov V.A., Leikina E., Bronk P., Zimmerberg J. 1998. The pathway of membrane fusion catalyzed by influenza hemagglutinin: Restriction of lipids, hemifusion, and lipidic fusion pore formation. J. Cell Biol. 140, 1369–1382.
Kuzmin P.I., Zimmerberg J., Chizmadzhev Y.A., Cohen F.S. 2001. A quantitative model for membrane fusion based on low-energy intermediates. Proc. Natl. Acad. Sci. USA. 98, 7235–7240.
Frolov V.A., Cho M.-S., Bronk P., Reese T.S., Zimmerberg J. 2000. Multiple local contact sites are induced by GPI-linked influenza hemagglutinin during hemifusion and flickering pore formation. Traffic. 1, 622–630.
Chernomordik L.V., Kozlov M.M., Zimmerberg J. 1995. Lipids in biological membrane fusion. J. Membr. Biol. 146, 1–14.
Chernomordik L.B., Melikyan G.B., Chizmadzhev Yu.A. 1987. Planar lipid bilayers as a model for studying fusion of biological membranes. Biologicheskie membrany (Rus.). 4, 117–164.
Leikin S.L., Kozlov M.M., Chernomordik L.V., Markin V.S., Chizmadzhev Yu.A. 1987. Membrane fusion: Overcoming of the hydration barrier and local restructuring. J. Theor. Biol. 129, 411–425.
Mueller M., Katsov K., Schick M. 2002. New mechanism of membrane fusion. J. Chem. Phys. 116, 2342–2345.
Frolov V.A., Dunina-Barkovskaya A.Y., Samsonov A.V., Zimmerberg J. 2003. Membrane permeability changes at early stages of influenza hemagglutinin-mediated fusion. Biophys. J. 85, 1725–1733.
Chernomordik L.V., Kozlov M.M. 2003. Protein-lipid interplay in fusion and fission of biological membranes. Annu. Rev. Biochem. 72, 175–207.
Chizmadzhev Yu. A. 2012. Membrane fusion. Biol. membrany. 29, 65–72.
Akimov S.A., Kuzmin P.I., Chizmadzhev Yu.A. 2002. Fusion model based on low-energy intermediates: Non-zero spontaneous curvature. Biologicheskie membrany (Rus.). 19, 264–272.
Rand R.P., Parsegian V.A. 1989. Hydration forces between phospholipid bilayers. Biochim. Biophys. Acta. 988, 351–376.
Rand R.P., Fuller N.L. 1994. Structural dimensions and their changes in a reentrant hexagonal-lamellar transition of phospholipids. Biophys. J. 66, 2127–2138.
Helfrich W. 1973. Elastic properties of lipid bilayers: Theory and possible experiments. Z. Naturforsch. 28c, 693–703.
Markin V.S., Albanesi J.P. 2002. Membrane fusion: Stalk model revisited. Biophys. J. 82, 693–712.
Marcelja S., Radic N. 1976. Repulsion of interfaces due to boundary water. Chem. Phys. Lett. 42, 129–132.
Lipowsky R. 1995. Handbook of biological physics. Ed. by R. Lipowsky and E. Sackmann. Elsevier Science B. Vol. 1.
Tatulian S.A., Gordeliy V.I., Sokolova A.E., Syrykh A.G. 1991. A neutron diffraction study of membrane interactions. Biochim. Biophys. Acta. 1070, 143–151.
Shcherbakov A.A., Chizmadhev Yu.A. 1996. Analysis of the conductance fluctuations during fusion pore evolution. Biologicheskie membrany (Rus.). 13, 330–336.
Rawicz W., Olbrich K.C., McIntosh T., Needham D., Evans E. 2000. Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys. J. 79, 328–339.
Fuller N., Rand R.P. 2001. The influence of lysolipids on the spontaneous curvature and bending elasticity of phospholipid membranes. Biophys. J. 81, 243–254.
Siegel D.P. 1993. Energetics of intermediates in membrane fusion: Comparison of stalk and inverted micellar intermediate mechanisms. Biophys. J. 65, 2124–2140.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © S.A. Akimov, R.J. Molotkovsky, T.R. Galimzyanov, A.V. Radaev, L.A. Shilova, P.I. Kuzmin, O.V. Batishchev, G.F. Voronina, Yu.A. Chizmadzhev, 2014, published in Biologicheskie Membrany, 2014, Vol. 31, No. 1, pp. 14–24.
Rights and permissions
About this article
Cite this article
Akimov, S.A., Molotkovsky, R.J., Galimzyanov, T.R. et al. Model of membrane fusion: Continuous transition to fusion pore with regard of hydrophobic and hydration interactions. Biochem. Moscow Suppl. Ser. A 8, 153–161 (2014). https://doi.org/10.1134/S1990747814010024
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747814010024