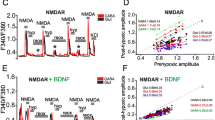
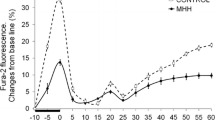
Abstract
It is known that brief episodes of hypoxia protect neurons from death caused by global ischemia and hypoxia (hypoxic preconditioning). At the same time, brief hypoxia may cause a phenomenon of posthypoxic hyperexcitability during reoxygenation, which can lead to the death of separate neurons due to their individual differences. In this work we compare the effects of short-term hypoxia on the initiation of preconditioning and posthypoxic hyperexcitability in two populations of neurons: inhibitory GABAergic neurons and excitatory glutamatergic neurons. Preconditioning effect was evaluated according to the suppression of the NMDA-receptor activity. The phenomenon of posthypoxic hyperexcitability was estimated by the appearance of spontaneous synchronized Ca2+ spikes in the neuronal network during reoxygenation after each episode of hypoxia. It is shown that the preconditioning effect occurs only in glutamatergic neurons. In the GABAergic neurons the effect of preconditioning was not observed. The activity of NMDA receptors in these neurons was not suppressed but increased after each episode of hypoxia. At the moment of posthypoxic synchronous Ca2+-spike generation, a global increase of the cytoplasmic Ca2+ concentration occurred in a few of GABAergic neurons, followed by the apoptotic death of these cells. The anti-inflammatory cytokine, interleukin-10 (IL-10) prevented the development of posthypoxic hyperexcitability, inhibiting spontaneous synchronous Ca2+ spike, and protected GABAergic neurons from the death, restoring the preconditioning effect in them. PI3-kinase inhibitors wortmannin and LY294002 prevented the IL-10 protective effect abolishing the inhibiting effect of IL-10 on the generation of the Ca2+ synchronous spike. These findings point out to the leading role of GABAergic neurons in the development of posthypoxic hyperexcitability. We suggest that the reason for posthypoxic hyperexcitability in the network is a weakening of the inhibiting effect of GABAergic neurons. Activation of different signaling pathways leading to activation of PKB- and PKG-dependent phosphorylation in the neurons of this type represents a possible strategy to protect neurons from death during hypoxia.
Similar content being viewed by others
Abbreviations
- NMDA:
-
N-methyl-D-aspartate
- GABA:
-
gamma-aminobutyric acid
- PI3K:
-
phosphoinositide 3-kinase
- DIV:
-
days in vitro
- U73122:
-
inhibitor of phospholipase C
- GAD65/67:
-
glutamate decarboxylase 65/67
- ROS:
-
reactive oxygen species
- NOS:
-
NO synthase
- PLC:
-
phospholipase C
- IP3:
-
inositol trisphosphate
References
Siesj B.K. 1986. Calcium and ischemic brain damage. Eur. Neurol. 25(1), 45–56.
Choi D.W. Glutamate neurotoxicity in cortical cell culture is calcium dependent. 1985. Neurosci. Lett. 58(3), 293–297.
Friedman L.K. 2006. Calcium: A role for neuroprotection and sustained adaptation. Mol. Intervations. 6(6), 315–329.
Heurteaux C., Lauritzen I., Widmann C., Lazdunski M. 1995. Essential role of adenosine, adenosine A1 receptors, and ATP-sensitive K+ channels in cerebral ischemic preconditioning. Proc. Natl. Acad. Sci. USA. 92, 4666–4670.
Kumral A., Baskin H., Gokmen N., Yilmaz O., Genc K., Genc S., Tatli M.M., Duman N., Ozer E., Ozkan H. 2004. Selective inhibition of nitric oxide in hypoxicischemic brain model in newborn rats: Is it an explanation for the protective role of erythropoietin? Biol. Neonate. 85, 51–54.
Bernaudin M., Tang Y., Reilly M., Petit E., Sharp F.R. 2002. Brain genomic response following hypoxia and re-oxygenation in the neonatal rat. Identification of genes that might contribute to hypoxia-induced ischemic tolerance. J. Biol. Chem. 277, 39728–39738.
Bergeron M., Gidday J.M., Yu A.Y., Semenza G.L., Ferriero D.M., Sharp F.R. 2000. Role of hypoxiainducible factor-1 in hypoxia-induced ischemic tolerance in neonatal rat brain. Ann. Neurol. 48, 285–296.
Bond A., Lodge D., Hicks C.A., Ward M.A., O’Neill M.J. 1999. NMDA receptor antagonism, but not AMPA receptor antagonism attenuates induced ischaemic tolerance in the gerbil hippocampus. Eur. J. Pharmacol. 380, 91–99.
Godukhin O.V., Savin A.S., Kalemenev S., Levin S. 2002. Neuronal hyperexcitability induced by repeated brief episodes of hypoxia in rat hippocampal slices: Involvement of ionotropic glutamate receptors and L-type Ca2+ channels. Neuropharmacol. 42, 459–466.
Levin S.G., Godukhin O.V. 2009. Comparative roles of ATP-sensitive K+ channels and Ca2+-activated BK+-channels in posthypoxic hyperexcitability and rapid hypoxic preconditioning in hippocampal CA1 pyramidal neurons in vitro. Neurosci. Lett. 461, 90–94.
Turovskaya M.V., Turovsky E.A., Zinchenko V.P., Levin S.G., Shamsutdinova A.A., Godukhin O.V. 2011. Repeated brief episodes of hypoxia modulate the calcium responses of ionotropic glutamate receptors in hippocampal neurons. Neurosci. Lett. 496, 11–14.
Turovskaya M.V., Turovsky E.A., Zinchenko V.P., Levin S.G., Godukhin O.V. 2012. Interleukin-10 modulates [Ca2+]i response induced by repeated NMDA receptor activation with brief hypoxia through inhibition of InsP3-sensitive internal stores in hippocampal neurons. Neurosci. Lett. 516(1), 151–155.
Bachis A., Colangelo A.M., Vicini S., Doe P.P., De Bernardi M.A., Brooker G., Mocchetti I. 2001. Interleukin-10 prevents glutamate-mediated cerebellar granule cell death by blocking caspase-3-like activity. J. Neurosci. 21(9), 3104–3112.
Qian L., Block M.L., Wei S.J., Lin C., Reece J., Pang H., Wilson B., Hong J.S., Flood P.M. 2006. Interleukin-10 protects lipopolysaccharide-induced neurotoxicity in primary midbrain cultures by inhibiting the function of NADPH oxidase. J. Pharmacol. Exp. Ther. 319(1), 44–52.
Sharma S., Yang B., Xi X., Grotta J.C., Aronowski J., Savitz S.I. 2011. IL-10 directly protects cortical neurons by activating PI-3 kinase and STAT-3 pathways. Brain Res. 1373, 189–194.
Belousov A.B. Godfraind J.M., Krnjevic K. 1995. Internal Ca2+ stores involved in anoxic responses of rat hippocampal neurons. J. Physiol. 486(3), 547–556.
Brewer G.J., Torricelli J.R., Evege E.K., Price P.J. 1993. Optimized survival of hippocampal neurons in B27-supplemental neurobasal, a new serum-free medium combination. J. Neurosci. Res. 35, 567–576.
Kononov A.V., Bal’ N.V., and Zinchenko V.P. 2012. Control of spontaneous synchronous Ca2+ oscillations in hippocampal neurons by GABAergic neurons containing kainate receptors without desensitization. Biochemistry (Moscow) Supplement Series A: Membrane and Cell Biology. 6(2), 215–220.
Kristian T., Siesjo B.K. 1998. Calcium in ischemic cell death. Stroke. 29, 705–718.
Bonde C., Noraberg J., Noer H., Zimmer J. 2005. Ionotropic glutamate receptors and glutamate transporters are involved in necrotic neuronal cell death induced by oxygenglucose deprivation of hippocampal slice cultures. Neurosci. 136(3), 779–794.
Rivollier A., Perrin-Cocon L., Luche S., Diemer H., Strub J.M., Hanau D., van Dorsselaer A., Lotteau V., Rabourdin-Combe C., Rabilloud T., Servet-Delprat C. 2006. High expression of antioxidant proteins in dendritic cells: Possible implications in atherosclerosis. Mol. Cell Proteomics. 5(4), 726–736.
Lynch G., Baudry M. 1984. The biochemistry of memory: A new and specific hypothesis. Science. 224(4653), 1057–1063.
Alexi T., Borlongan C.V., Faull R.L.M., Williams C.E., Clark R.G., Gluckman P.D., Hughes P.E. 2000. Neuroprotective strategies for basal ganglia degeneration: Parkinson’s and Huntington’s diseases. Progr. Neurobiol. 60, 409–470.
Simonian N.A., Coyle J.T. 1996. Oxidative stress in neurodegenerative diseases. Annu. Rev. Pharmacol. Toxicol. 36, 83–106.
Cassarino D.S., Bennett J.P. Jr. 1999. An evaluation of the role of mitochondria in neurodegenerative diseases: Mitochondrial mutations and oxidative pathology, protective nuclear responses, and cell death in neurodegeneration. Brain Res. Rev. 29(1), 1–25.
Kruman I., Bruce-Keller A.J., Bredesen D., Waeg G., Mattson M.P. 1997. Evidence that 4-hydroxynonenal mediates oxidative stress-induced neuronal apoptosis. J. Neurosci. 17(13), 5089–5100.
Choi D.W., Peters S., Viseskul V. 1987. Dextrorphan and levorphanol selectively block N-methyl-D-aspartate receptor-mediated neurotoxicity on cortical neurons. J. Pharmacol. Exp. Ther. 242(2), 713–720.
Perez-Pinzon M.A., Mumford P.L., Rosenthal M. 1996. Anoxic preconditioning in hippocampal slices: Role of adenosine. Neurosci. 75, 687–694.
Tremblay R., Chakravarthy B., Hewitt K., Tauskela J., Morley P., Atkinson T., Durkin J.P. 2000. Transient NMDA receptor inactivation provides long-term protection to cultured cortical neurons from a variety of deaths. J. Neurosci. 20(19), 7183–7192.
Liu J., Ginis I., Spatz M., Hallenbeck J.M. 2000. Hypoxic preconditioning protects cultured neurons against hypoxic stress via TNF-α and ceramide. Am. J. Physiol. Cell. Physiol. 278(1), 144–153.
Wu L.Y., Ding A.S., Zhao T., Ma Z.M., Wang F.Z., Fan M. 2005. Underlying mechanism of hypoxic preconditioning decreasing apoptosis induced by anoxia in cultured hippocampal neurons. Neurosignals. 14(3), 109–116.
Sharp F.R., Ran R., Lu A., Tang Y., Strauss K.I., Glass T., Ardizzone T., Bernaudin M. 2004. Hypoxic preconditioning protects against ischemic brain injury. NeuroRx. 1(1), 26–35.
Jia J., Wang X., Li H., Han S., Zu P., Li J. 2007. Activations of nPKCe and ERK1/2 were involved in oxygenglucose deprivation-induced neuroprotection via NMDA receptors in hippocampal slices of mice. J. Neurosurg. Anesthesiol. 19(1), 18–24.
Gidday J.M., Shah A.R., Maceren R.G., Wang Q., Pelligrino D.A., Holtzman D.M., Park T.S. 1999. Nitric oxide mediates cerebral ischemic tolerance in a neonatal rat model of hypoxic preconditioning. J. Cereb. Blood Flow Metab. 19, 331–340.
Gonzalez-Zulueta M., Feldman A.B., Klesse L.J., Kalb R.G., Dillman J.F., Parada L.F., Dawson T.M., Dawson V.L. 2000. Requirement for nitric oxide activation of p21(ras)/extracellular regulated kinase in neuronal ischemic preconditioning. Proc. Natl. Acad. Sci. USA. 97, 436–441.
Gonzalez-Burgos G., Lewis D.A. 2008. GABA-neurons and the mechanisms of network oscillations: Implications for understanding cortical dysfunction in schizophrenia. Schizophr. Bull. 34(5), 944–961.
Earls L.R., Hacker M.L., Watson J.D., Miller D.M. 3rd. 2010. Coenzyme Q protects Caenorhabditis elegans GABA neurons from calcium-dependent degeneration. Proc. Natl. Acad. Sci. USA. 107(32), 14460–14465.
Romijn H.J. 1989. Preferential loss of GABAergic neurons in hypoxia-exposed neocortex slab cultures is attenuated by the NMDA receptor blocker D-2-amino-7-phosphonoheptanoate. Brain Res. 501(1), 100–104.
Romijn H.J., Ruijter J.M., Wolters P.S. 1988. Hypoxia preferentially destroys GABAergic neurons in developing rat neocortex explants in culture. Exp. Neur. 100(2), 332–340.
Nitsch C., Scotti A., Sommacal A., Kalt G. 1989. GABAergic hippocampal neurons resistant to ischemia-induced neuronal death contain the Ca2+-binding protein parvalbumin. Neurosci. Lett. 105(3), 263–268.
Levin S.G., Godukhin O.V. 2011. Anti-inflammatory cytokines, TGF-β1 and IL-10, exert anti-hypoxic action and abolish posthypoxic hyperexcitability in hippocampal slice neurons: Comparative aspects. Exp. Neur. 232, 329–332.
Flanders K.C., Ren R.F., Lippa C.F. 1998. Transforming growth factor-βs in neurodegenerative disease. Progr. Neurobiol. 54(1), 71–85.
Sharma S., Yang B., Xi X., Grotta J.C., Aronowski J., Savitz S.I. 2011. IL-10 directly protects cortical neurons by activating PI3 kinase and STAT-3 pathways. Brain. Res. 1373, 189–194.
Müller M., Felmy F., Schwaller B., Schneggenburger R. 2007. Parvalbumin is a mobile presynaptic Ca2+ buffer in the calyx of held that accelerates the decay of Ca2+ and short-term facilitation. J. Neurosci. 27(9), 2261–2271.
Ha K.S., Kim K.M., Kwon Y.G., Bai S.K., Nam W.D., Yoo Y.M., Kim P.K., Chung H.T., Billiar T.R., Kim Y.M. 2003. Nitric oxide prevents 6-hydroxydopamine-induced apoptosis in PC12 cells through cGMP-dependent PI3 kinase/Akt activation. FASEB J. 17, 1036–1047.
Szado T., Vanderheyden V., Parys J.B., De Smedt H., Rietdorf K., Kotelevets L. 2008. Phosphorylation of inositol 1,4,5-trisphosphate receptors by protein kinase B/Akt inhibits Ca2+ release and apoptosis. Proc. Natl. Acad. Sci. USA. 105, 2427–2432.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © M.V. Turovskaya, E.A. Turovsky, A.V. Kononov, V.P. Zinchenko, 2013, published in Biologicheskie Membrany, 2013, Vol. 30, No. 5–6, pp. 479–490.
Rights and permissions
About this article
Cite this article
Turovskaya, M.V., Turovsky, E.A., Kononov, A.V. et al. Short-term hypoxia induces a selective death of GABAergic neurons. Biochem. Moscow Suppl. Ser. A 8, 125–135 (2014). https://doi.org/10.1134/S199074781305019X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S199074781305019X