Abstract
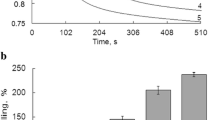
Effects of different inhibitors of lipid peroxidation (LP), such as sulphur-containing oligoquinone hypoxen, natural flavonoid dihydroquercetin (DHQ), and β-ionol, on Ca2+-induced calcium release from rat liver mitochondria (RLM) were investigated during oxidation of various substrates. The hypothesis about interrelation between antioxidant properties and influence of selected substances on spontaneous calcium release from mitochondria was verified. Degree of antioxidant activity of the selected substances was estimated by the inhibition of LP induced by Fe2+/ATP complex in phospholipid emulsion or in rat liver mitochondria (RLM). According to the inhibition efficacy the investigated substances were ordered as follows: β-ionol ≫ hypoxen > DHQ. 50% inhibition of oxygen consumption during LP of phospholipid emulsion was reached in presence of 3.2 ± 0.6 μM of β-ionol, 15.0 ± 1.1 μM of hypoxen, or 19.8 ± 1.7 μM of DHQ. Among the investigated antioxidants hypoxen only decreased spontaneous release of calcium from RLM after calcium accumulation by RLM. The impact of the antioxidants onto calcium current depended on the oxidized substrate. Hypoxen effect was most expressed during the oxidation of NAD-dependent substrate. The direct relationship between the antioxidant activity of the selected antioxidants and their influence on calcium transport in RLM was not revealed. The results indicate that the choice of antiischemic preparations should not only rely on their antioxidant activities.
Similar content being viewed by others
Abbreviations
- Mt:
-
mitochondria
- MPTP:
-
mitochondrial permeability transition pore
- RLM:
-
rat liver mitochondria
- LP:
-
lipid peroxidation
- DHQ:
-
dihydroquercetin
- mCICR:
-
Ca2+-induced calcium release from mitochondria
- Suc:
-
succinate
- Glu:
-
glutamate
- HB:
-
3-hydroxybutirate
- OG:
-
2-oxoglutarate
- Mal:
-
malate
References
Crompton, M., The Mitochondrial Permeability Transition Pore and Its Role in Cell Death, Biochem. J., 1999, vol. 341, pp. 233–249.
Bernardi, P., Mitochondrial Transport of Cations: Channels, Exchangers, and Permeability Transition, Physiol. Rev., 1999, vol. 79, pp. 1127–1155.
Zoratti, M. and Szabo, I., The Mitochondrial Permeability Transition, Biochim. Biophys. Acta, 1995, vol. 1241, no. 2, pp. 139–176.
Green, D.R. and Kroemer, G., The Pathophysiology of Mitochondrial Cell Death, Science, 2004, vol. 305, pp. 626–629.
Weiss, J.N., Korge, P., Honda, H.M., and Ping, P., Role of the Mitochondrial Permeability Transition in Myocar dial Disease, Circulation Research, 2003, vol. 93, pp. 292–301.
Di Lisa, F. and Bernardi, P., Mitochondrial Function and Myocardial Aging. A Critical Analysis of the Role of Permeability Transition, Cardiovasc. Res., 2005, vol. 66, pp. 222–232.
Greene, E.L. and Paller, M.S., Calcium and Free Radicals in Hypoxia/Reoxygenation Injury of Renal Epithelial Cells, Am. J. Physiol. Renal Physiol., 1994, vol. 266, pp. F13–F20.
Richter, C. and Kass, G.E.N., Oxidative Stress in Mitochondria: Its Relationship to Cellular Ca2+ Homeostasis, Cell Death, Proliferation, and Differentiation, Chem. Biol. Interaction, 1991, vol. 77, pp. 1–23.
Ganitkevich, V.Y., The Role of Mitochondria in Cytoplasmic Ca2+ Cycling, Exp. Physiol., 2003, vol. 88, pp. 91–97.
Chien, K.R., Han, A., Sen, A., Buja, L.M., and Willerson, J.T., Accumulation of Unesterified Arachidonic Acid in Ichemic Canine Myocardium. Relationship to a Phosphatidylcholine Deacylation-Reacylation Cycle and the Depletion of Membrane Phospholipids, Circ. Res., 1984, vol. 54, pp. 313–322.
Portilla, D., Mandel, L.J., Bar-Sagi, D., and Millington, D.S., Anoxia Induces Phospholipase A2 Activation in Rabbit Renal Proximal Tubules, Am. J. Physiol., 1992, vol. 262, pp. F354–F360.
Das, D.K., Engelman, R.M., Rousou, J.A., et al., Role of Membrane Phospholipids in Myocardial Injury Induced by Ischemia and Reperfusion, Am. J. Physiol., 1986, vol. 251, no. 20, pp. H71–H79.
Kowaltowski, A.J., Castilho, R.F., and Vercesi, A.E., Mitochondrial Permeability Transition and Oxidative Stress, FEBS Lett., 2001, vol. 495, nos. 1–2, pp. 12–15.
Vercesi, A.E., Kowaltowski, A.J., Oliveira, H.C., and Castilho, R.F., Mitochondrial Ca2+ Transport, Permeability Transition and Oxidative Stress in Cell Death: Implications in Cardiotoxicity, Neurodegeneration and Dyslipidemias, Front Biosci., 2006, vol. 11, pp. 2554–2564.
Richter, C. and Kass, G.E.N., Oxidative Stress In Mitochondria: Its Relationship to Cellular Ca2+ Homeostasis, Cell Death, Proliferation, and Differentiation, Chem. Biol. Interaction, 1991, vol. 77, pp. 1–23.
Fontaine, E. and Bernardi, P., Progress on the Mitochondrial Permeability Transition Pore: Regulation by Complex I and Ubiquinone Analogs, J. Bioenerg. Biomembr., 1999, vol. 31, pp. 335–345.
Steenbergen, C., Murphy, E., Watts, J.A., and London, R.E., Correlation between Cytosolic Free Cacium, Contracture ATP and Irreversible Ischemic Injury in Perfused Rat Heart, Circ. Res., 1990, vol. 66, pp. 135–146.
Papa, S. and Skulachev, V.P., Reactive Oxygen Species, Mitochondria, Apoptosis and Aging, Mol. Cell Biochem., 1997, vol. 174, pp. 305–319.
Turrens, J.F., Mitochondrial Formation of Reactive Oxygen Species, J. Physiol., 2003, vol. 552, pp. 335–344.
Richter, C. and Frei, B., Ca2+ Release from Mitochondria Induced by Prooxidants, Free Radic. Biol. Med., 1988, vol. 4, pp. 365–375.
James, A.M., Smith, R.A.J., and Murphy, M.P., Antioxidant and Prooxidant Properties of Mitochondrial Coenzyme Q, Arch. Biochem. Biophys., 2004, vol. 423, pp. 47–56.
Fontaine, E., Ichas, F., and Bernardi, P., A Ubiquinone-Binding Site Regulates the Mitochondrial Permeability Transition Pore, J. Biol. Chem., 1998, vol. 273, no. 40, pp. 25734–25740.
Walter, L., Nogueira, V., Leverve, X., Heitz, M.-P., Bernardi, P., and Fontaine, E., Three Classes of Ubiquinone Analogs Regulate the Mitochondrial Permeability Transition Pore Through a Common Site J. Biol. Chem., 2000, vol. 275, pp. 29521–29527.
Abreu, R., Santos, D., and Moreno, A., Effects of Carvedilol and Its Analog BM-910228 on Mitochondrial Function and Oxidative Stress, J. Pharm. Exp. Therap., 2000, vol. 295, no. 3, pp. 1022–1030.
Okovity, S.V. and Smirnov, A.V., Antihypoxants, Eksperim. Klinich. Farmakol. (Rus.), 2001, vol. 64, no. 3, pp. 76–80.
Smirnov, V.S. and Kuz’mich, M.K., Hypoksen (Hypoxen), St. Petersburg—Moscow, FARMindex, 2001.
Uminky, A.A., Havsteen, B.H., and Bakaneva, V.F., Biokhimia flavonoidov i ikh znachenie v meditsine (Biochemistry and Medical Significance of Flavonoids), Pushchino, Photon-vek Company, 2007.
Vladimirov, Yu.A. and Archakov, A.I., Perekisnoe okislenie lipidov v biologicheskikh membranakh (Lipid Peroxidation in Biological Membranes), Moscow, Nauka, 1972.
Sassa, H., Takaisi, Y., and Terada, H., The Triterpene Celastrol as a Very Potent Inhibitor of Lipid Peroxidation in Mitochondria, Biochem. Biophys. Res. Commun., 1990, vol. 172, pp. 890–897.
Eaton, J.W. and Qian, M., Molecular Bases of Cellular Iron Toxicity, Free Rad. Biol. Med., 2002, vol. 32, no. 9, pp. 833–840.
Bodrova, M.E., Dedukhova, V.I., and Mokhova, E.N., Generation of Transmembrane Electrical Potential during NADH Oxidation via the External Pathway and the Fatty Acid Uncoupling Effect after Transient Opening of the Ca2+-Dependent Cyclosporin A-Sensitive Pore in Liver Mitochondria, Biokhimia, (Rus.), 2000, vol. 65, no. 4, pp. 562–569 [Transl. version in Biochemistry (Moscow), 2000, vol. 65, no. 4, pp 477–484].
Praktikum po biokhimii (Handbook on Biochemistry), Meschkova, N.P. and Severin, S.E., Eds., Moscow, Moscow University Publishing House, 1979, pp. 90–91.
Kuznetsova, I.N. and Maevsky, E.I., Emulsion of Perfluorocompound for Medical Use and Method for Its Production, Russian Patent no. 2259819, Bull. 25 (10.09.2005).
Budnikov, E.Iu., Postnov, A.Iu., Afanas’eva, G.V., Doroshchuk, A.D., Bus’ko, E.V., and Postnov, Iu.V., Calcium Induced Calcium Release from Liver Mitochondria of Spontaneously Hypertensive Rats, Kardiologiia (Rus.), 2005, vol. 45, no. 7, pp. 49–53.
Zaitsev, V.G., Ostrovskii, O.V., and Zakrevskii, V.I., Correlation between Chemical Structure and a Target as Basis for Classification of Direct-Acting Antioxidants, Eksp. Klin. Farmakol., (Rus.), 2003, vol. 66, no. 4, pp. 66–70.
Huang, X., Zhai, D., and Huang, Y., Study on the Relationship between Calcium-Induced Calcium Release from Mitochondria and PTP Opening, Mol. Cell Biochem., 2000, vol. 213, pp. 29–35.
Grishina, E.V., Katuzan, Y.V., Popov, V.G., Kuz’mich, M.K., and Maevskii, E.I., About the Possible Mechanism of Antihypoxic Action of Hypoxen, Horizons of Biophysics, Ivanitskii, G.R., Ed., Pushchino, 2003, pp. 120–123.
Grishina, E.V., Khaustova, Y.V., Pogorelova, V.G., Pogorelov, A.G., Kuz’mich, M.K., and Maevskii, E.I., Accelerated Utilization of Lactate under the Effect of Hypoxen after Intensive Exercise, Bull. Exp. Biol. Med. (Rus.), 2008, vol. 145, pp. 158–161 [Transl. version in Bull. Exp. Biol. Med., 2008, vol. 145, no. 2, pp. 198–201.]
Radi, R., Turrens, J.F., and Freeman, B.A., Cytochrome C-Catalyzed Membrane Lipid Peroxidation by Hydrogen Peroxide, Arch. Biochem. Biophys., 1991, vol. 288, no. 1, pp. 118–125.
Rhee, S.G., Kim, K.H., Chae, H.Z., Yim, M.B., Uchida, K., Netto, L.E., and Stadtman, E.R., Antioxidant Defense Mechanisms, a New Thiol-Specific Antioxidant Enzyme, Ann. NY Acad. Sci., 1994, vol. 738, pp. 86–92.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.V. Grishina, Y.V. Khaustova, E.I. Maevsky, 2009, published in Biologicheskie Membrany, 2009, Vol. 26, No. 6, pp. 505–513.
The article was translated by the authors.
Rights and permissions
About this article
Cite this article
Grishina, E.V., Khaustova, Y.V. & Maevsky, E.I. Inhibition of Ca-induced calcium release from mitochondria by antiischemic phenol-based antioxidants. Biochem. Moscow Suppl. Ser. A 3, 459–466 (2009). https://doi.org/10.1134/S199074780904014X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S199074780904014X