Abstract
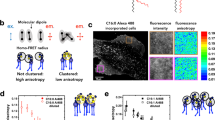
Gangliosides are significant participants in suppression of immune system during tumor processes. It was shown that they can induce apoptosis of T-lymphocytes in a raft-dependent manner. Fluorescence confocal microscopy was used to study distribution and influence of ganglioside GM1 on raft properties in giant unilamellar vesicles. Both raft and non-raft phase markers were utilized. No visible phase separation was observed without GM1 unless lateral tension was applied to the membrane. At 2 mol % of GM1 large domains appeared indicating macroscopic phase separation. Increase of GM1 content to 5 mol % resulted in shape transformation of the domains consistent with growth of line tension at the domain boundary. At 10 mol % of GM1 almost all domains were pinched out from vesicles, forming their own homogeneous liposomes. Estimations showed that the change of the GM1 content from 2 to 5–10 mol % resulted in a several-fold increase of line tension. This finding provides a possible mechanism of apoptosis induction by GM1. Incorporation of GM1 into a membrane leads to an increase of the line tension. This results in a growth of the average size of rafts due to coalescence or merger of small domains. Thus, necessary proteins can find themselves in one common raft and start the corresponding cascade of reactions.
Similar content being viewed by others
References
Igney, F.H. and Krammer, P.H., Death and Anti-Death: Tumor Resistance to Apoptosis, Nat. Rev. Cancer, 2002, vol. 2, pp. 277–288.
Birkle, S., Zeng, G., Gao, L., Yu, R.K., and Aubry, J., Role of Tumor-Associated Gangliosides in Cancer Progression, Biochimie, 2003, vol. 85, pp. 455–463.
Ladisch, S., Kitada, S., and Hays, E.F., Gangliosides Shed by Tumor Cells Enhance Tumour Formation in Mice, J. Clin. Invest., 1987, vol. 79, pp. 1879–1882.
Heitger, A. and Ladisch, S., Gangliosides Block Antigen Presentation by Human Monocytes, Biochim. Biophys. Acta, 1996, vol. 1303, pp. 161–168.
Hueber, A.O., Bernard, A.M., Herincs, Z., Couzinet, A., and He, H.T., An Essential Role for Membrane Rafts in the Initiation of Fas/CD95-Triggered Cell Death in Mouse Thymocytes, EMBO Rep., 2002, vol. 3, pp. 190–196.
Grassme, H., Jekle, A., Riehle, A., Schwarz, H., Berger, J., Sandho, K., Kolesnick, R., and Gulbins, E., CD95 Signaling via Ceramide Rich Membrane Rafts, J. Biol. Chem., 2001, vol. 276, pp. 20589–20596.
Burek, C., Roth, J., Koch, H.G., Harzer, K., Los, M., and Shlutze-Osthoff, K., The Role of Ceramide in Receptor- and Stress-Induced Apoptosis Studied in Acidic Ceramidase Farber Desease Cells, Oncogene, 2001, vol. 20, pp. 6493–6502.
Hartel, S., Fanani, M.L., and Maggio, B., Shape Transitions and Lattice Structuring of Ceramide-Enriched Domains Generated by Sphingomielinase in Lipid Monolayers, Biophys. J., 2005, vol. 88, pp. 287–304.
London, E., Insights into Lipid Raft Structure and Formation from Experiments in Model Membranes, Curr. Opin. Struct. Biol., 2002, vol. 12, pp. 480–486.
Brown, D.A. and London, E., Structure and Origin of Ordered Lipid Domains in Biological Membranes, J. Membr. Biol., 1998, vol. 164, pp. 103–114.
Brown, D.A. and London, E., Functions of Lipid Rafts in Biological Membranes, Annu. Rev. Cell. Dev. Biol., 1998, vol. 14, pp. 111–136.
Brown, D.A. and London, E., Structure and Function of Sphingolipid- and Cholesterol-Rich Membrane Rafts, J. Biol. Chem., 2000, vol. 275, pp. 17221–17224.
Simons, K. and Ikonen, E., Functional Rafts in Cell Membranes, Nature, 1997, vol. 387, pp. 569–572.
Molotkovskaya, I.M., Kholodenko, R.V., and Molotkovsky, J.G., Influence of Gangliosides on the IL-2- and IL-4-Dependent Cell Proliferation, Neurochem. Res., 2002, vol. 27, pp. 761–770.
Boldyrev, I.A., Zhai, X., Momsen, H.L., Brockman, H.L., Brown, R.E., and Molotkovsky, J.G., New Bodipy Lipid Probes for Fluorescence Studies of Membranes, J. Lipid Res., 2007, vol. 48, pp. 1518–1532.
Marushchak, D., Gretskaya, N., Mikhalyov, I., and Johansson, L.B.-A., Self-Aggregation — an Intrinsic Property of GM1 in Lipid Bilayers, Mol. Membr. Biol., 2007, vol. 24, pp. 102–112.
Tanaka, T., Tamba, Y., Masum, S.M., Yamashita, Y., and Yamazaki, M., La3+ and Gd3+ Induce Shape Change of Giant Unilamellar Vesicles of Phosphatidylcholine, Biochim. Biophys. Acta, 2002, vol. 1564, pp. 173–182.
Ishitsuka, R., Yamaji-Hasegawa, A., Makino, A., Hirabayashi, Y., and Kobayashi, T.A., Lipid-Specific Toxin Reveals Heterogeneity of Sphingomyelin-Containing Membranes, Biophys. J., 2004, vol. 86, pp. 296–307.
Ayuyan, A.G. and Cohen, F.S., Lipid Peroxides Promote Large Rafts: Effects of Excitation of Probes in Fluorescence Microscopy and Electrochemical Reactions during Vesicle Formation, Biophys. J., 2006, vol. 91, pp. 2172–2183.
Ayuyan, A.G. and Cohen, F.S., Raft Composition at Physiological Temperature and pH in the Absence of Detergents, Biophys. J., 2008, vol. 94, pp. 2654–2666.
Samsonov, A.V., Mihalyov, I., and Cohen, F.S., Characterization of Cholesterol-Sphingomyelin Domains and Their Dynamics in Bilayer Membranes, Biophys. J., 2001, vol. 81, pp. 1486–1500.
Molotkovskaya, I.M., Svirshchevskaya, E.V., Litvinov, I.V., Mikhalyov, I.I., Dyatlovitskaya, E.V., Molotkovsky, J.G., and Bergelson, L.D., Immunosippressive Activity of Glycosphingolipids: A Study of the Interaction of Interleukin-2 with Gangliosides Using Cells and Model Systems, Biol. Membr., 1992, vol. 6, pp. 169–181.
Yuan, C. and Johnston, L.J., Distribution of Ganglioside GM1 in L-Alpha-Dipalmitoylphosphatidylcholine/Cholesterol Monolayers: A Model for Lipid Rafts, Biophys. J., 2000, vol. 79, pp. 2768–2781.
Dietrich, C., Volovyk, Z.N., Levi, M., Thompson, N.L., and Jacobson, K., Partitioning of Thy-1, GM1, and Cross-Linked Phospholipid Analogs into Lipid Rafts Reconstituted in Supported Model Membrane Monolayers, Proc. Natl. Acad. Sci. USA, 2001, vol. 98, pp. 10642–10647.
Baumgart, T., Hess, S.T., and Webb, W.W., Imaging Coexisting Fluid Domains in Biomembrane Models Coupling Curvature and Line Tension, Nature, 2003, vol. 425, pp. 821–824.
Veatch, S.L., Polozov, I.V., Gawrisch, K., and Keller, S.L., Liquid Domains in Vesicles Investigated by NMR and Fluorescence Microscopy, Biophys. J., 2004, vol. 86, pp. 2910–2922.
Akimov, S.A., Kuzmin, P.I., Zimmerberg, J., and Cohen, F.S., Lateral Tension Increases the Line Tension between Two Domains in a Lipid Bilayer Membrane, Phys. Rev. E., 2007, vol. 75, pp. 011919-1–011919-18.
Frolov, V.A.J., Chizmadzhev, Y.A., Cohen, F.S., and Zimmerberg, J., “Entropic Traps” in the Kinetics of Phase Separation in Multicomponent Membranes Stabilize Nanodomains, Biophys. J., 2006, vol. 91, pp. 189–205.
Evans, E., Heinrich, V., Ludwig, F., and Rawicz, W., Dynamic Tension Spectroscopy and Strength of Biomembranes, Biophys. J., 2003, vol. 85, pp. 2342–2350.
Bacia, K., Schwille, P., and Kurzchalia, T., Sterol Structure Determines the Separation of Phases and the Curvature of the Liquid-Ordered Phase in Model Membranes, Proc. Natl. Acad. Sci. USA, 2005, vol. 102, pp. 3272–3277.
Yuan, C., Furlong, J., Burgos, P., and Johnston, L.J., The Size of Lipid Rafts: An Atomic Force Microscopy Study of Ganglioside GM1 Domains in Sphingomyelin/DOPC/Cholesterol Membranes, Biophys. J., 2002, vol. 82, pp. 2526–2535.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Akimov, S.A., Hlaponin, E.A., Bashkirov, P.V. et al. Ganglioside GM1 increases line tension at raft boundary in model membranes. Biochem. Moscow Suppl. Ser. A 3, 216–222 (2009). https://doi.org/10.1134/S1990747809020159
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747809020159