Abstract
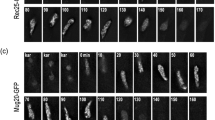
According to the radial loop model of chromosome organization, a major role in the formation and maintenance of chromosomes is played by the residual structures (the nuclear matrix in interphase nuclei and the chromosome scaffold in metaphase chromosomes). However, in vivo microscopy has recently revealed that the components of these “static” structures are highly mobile and continuously exchanged between specific target sites and the nucleoplasm or cytoplasm. This contradiction between predicted stability and observed dynamics led us to reexamine the principles underlying the association of proteins with residual structures. In the present paper, we have analyzed the association of two perichromosomal layer proteins, pKi-67 and B23, with the residual structures. The results show that these two proteins are associated with residual structures throughout the cell cycle; only those structures change that contain proteins precipitated by 2 M NaCl (nucleoli, perichromosomal layer, prenucleolar bodies, cytoplasm of mitotic cells). Both pKi-67 and B23 remain associated with the nuclear matrix even when they are translocated to nucleoplasmic foci due to inhibitor action or hypotonic treatment. However, in most cases it remains possible to extract a structurally visible protein fraction with 2 M NaCl (protein distributed in nucleoplasm). One may suppose that the protein fraction associated with residual structures includes molecules interacting with their binding sites at the moment of permeabilization, while the free proteins are extracted (i.e., during the interaction with binding sites, these proteins form salt-resistant complexes; however, on diffusion the same proteins are extractable by the high-salt solution). The residual structures may be considered as a “snapshot” of all proteins transiently (or statically) bound to their target sites at the moment of permeabilization.
Similar content being viewed by others
References
Gorski, S.A., Dundr, M., and Misteli, T., The Road Much Traveled: Trafficking in the Cell Nucleus, Curr. Opin. Cell Biol., 2006, vol. 18, pp. 284–290.
Harold, F.M., Molecules into Cells: Specifying Spatial Architecture, Microbiol. Mol. Biol. Rev., 2005, vol. 69, pp. 544–564.
Misteli, T., Physiological Importance of RNA and Protein Mobility in the Cell Nucleus, Histochem. Cell Biol., 2008, vol. 129, pp. 5–11.
Karsenti, E., Self-Organization in Cell Biology: a Brief History, Nat. Rev. Mol. Cell Biol., 2008, vol. 9, pp. 255–262.
Tsutsui, K.M., Sano, K., and Tsutsui, K., Dynamic View of the Nuclear Matrix, Acta Medica Okayama, 2005, vol. 59, pp. 113–120.
Sheval, E.V. and Polyakov, V.Y., Chromosome Scaffold and Structural Integrity of Mitotic Chromosomes, Russian J. Dev. Biol., 2006, vol. 37, pp. 337–349.
Černá, A., López-Fernández, C., Fernández, J.L., Moreno Díaz de la Espina, S., de la Torre, C., and Gosálvez, J., Triplex Configuration in the Nick-Free DNAs That Constitute the Chromosomal Scaffolds in Grasshopper Spermatids, Chromosoma, 2008, vol. 117, pp. 15–24.
Razin, S.V., Chromosomal DNA Loops May Constitute Basic Units of the Eukaryotic Genome Organization and Evolution, Crit. Rev. Eukaryot. Gene Expr., 1999, vol. 9, pp. 279–283.
Paulson, J.R. and Laemmli, U.K., The Structure of Histone Depleted Metaphase Chromosomes, Cell, 1977, vol. 12, pp. 817–828.
Hadlaczky, G., Sumner, A.T., and Ross, A., Protein-Depleted Chromosomes. I. Structure of Isolated Protein-Depleted Chromosomes, Chromosoma, 1981, vol. 81, pp. 537–555.
Maeshima, K. and Laemmli, U.K., A Two-Step Scaffolding Model for Mitotic Chromosome Assembly, Dev. Cell, 2003, vol. 4, pp. 467–480.
Kireeva, N., Lakonishok, M., Kireev, I., Hirano, T., and Belmont, A.S., Visualization of Early Chromosome Condensation: a Hierarchical Folding, Axial Glue Model of Chromosome Structure, J. Cell Biol., 2004, vol. 166, pp. 775–785.
Christensen, M.O., Larsen, M.K., Barthelmes, H.U., Hock, R., Andersen, C.L., Kjeldsen, E., Knudsen, B.R., Westergaard, O., Boege, F., and Mielke, C., Dynamics of Human DNA Topoisomerases IIα and IIβ in Living Cells, J. Cell Biol., 2002, vol. 157, pp. 31–44.
Tavormina, P.A., Come, M.G., Hudson, J.R., Mo, Y.Y., Beck, W.T., and Gorbsky, G.J., Rapid Exchange of Mammalian Topoisomerase IIα at Kinetochores and Chromosome Arms in Mitosis, J. Cell Biol., 2002, vol. 158, pp. 23–29.
Gerlich, D., Hirota, T., Koch, B., Peters, J.M., and Ellenberg, J., Condensin I Stabilizes Chromosomes Mechanically Through a Dynamic Interaction in Live Cells, Curr. Biol., 2006, vol. 16, pp. 333–344.
Oliveira, R.A., Heidmann, S., and Sunkel, C.E., Condensin I Binds Chromatin Early in Prophase and Displays a Highly Dynamic Association with Drosophila Mitotic Chromosomes, Chromosoma, 2007, vol. 116, pp. 259–274.
Poirier, M.G. and Marko, J.F., Mitotic Chromosomes are Chromatin Networks without a Mechanically Contiguous Protein Scaffold, Proc. Natl. Acad. Sci. USA, 2002, vol. 99, pp. 15393–15397.
Almagro, S., Riveline, D., Hirano, T., Houchmandzadeh, B., and Dimitrov, S., The Mitotic Chromosome is an Assembly of Rigid Elastic Axes Organized by Structural Maintenance of Chromosomes (SMC) Proteins and Surrounded by a Soft Chromatin Envelope, J. Biol. Chem., 2004, vol. 279, pp. 5118–5126.
Marko, J.F., Micromechanical Studies of Mitotic Chromosomes, Chromosome Res., 2008, vol. 16, pp. 469–497.
Sheval, E.V. and Polyakov, V.Y., Visualization of the Chromosome Scaffold and Intermediates of Loop Domain Compaction in Extracted Mitotic Cells, Cell Biol. Int., 2006, vol. 30, pp. 1028–1040.
Verheijen, R., Kuijpers, H.J., van Driel, R., Beck, J.L., van Dierendonck, J.H., Brakenhoff, G.J., and Ramaekers, F.C., Ki-67 Detects a Nuclear Matrix-Associated Proliferation-Related Antigen. II. Localization in Mitotic Cells and Association with Chromosomes, J. Cell Sci., 1989, vol. 92, pp. 531–540.
Belyaev, N.D., Budker, V.G., Dubrovskaya, V.A., Kim, A.A., Kiseleva, E.V., and Sidorov, V.N., Localization of Proteins Forming the Outer Surface of Isolated Metaphase Chromosomes, FEBS Lett., 1992, vol. 297, pp. 43–45.
Vega-Salas, D.E., Fernandez, M., and Nouri, K., Characterization and Changes of a Chromosomal-Scaffolding Protein in Human Epithelia, Cell Tissue Res., 2002, vol. 308, pp. 193–203.
Gassmann, R., Henzing, A.J., and Earnshaw, W.C., Novel Components of Human Mitotic Chromosomes Identified by Proteomic Analysis of the Chromosome Scaffold Fraction, Chromosoma, 2005, vol. 113, pp. 385–397.
Sheval, E.V. and Polyakov, V.Y., The Peripheral Chromosome Scaffold, a Novel Structural Component of Mitotic Chromosomes, Cell Biol. Int., 2008, vol. 32, pp. 708–712.
Tsutsui, K., Tsutsui, K., Sano, K., Kikuchi, A., and Tokunaga, A.J., Involvement of DNA Topoisomerase IIβ in Neuronal Differentiation, J. Biol. Chem., 2001, vol. 276, pp. 5769–5778.
Boisvert, F.M., Hendzel, M.J., and Bazett-Jones, D.P., Promyelocytic Leukemia (PML) Nuclear Bodies are Protein Structures That Do Not Accumulate RNA, J. Cell Biol., 2000, vol. 148, pp. 283–292.
Gilerovitch, H.G., Bishop, G.A., King, J.S., and Burry, R.W., The Use of Electron Microscopic Immunocytochemistry with Silver-Enhanced 1.4-nm Gold Particles to Localize GAD in the Cerebellar Nuclei, J. Histochem. Cytochem., 1995, vol. 43, pp. 337–343.
Sheval, E.V., Polzikov, M.A., Olson, M.O.J., and Zatsepina, O.V., A Higher Concentration of an Antigen within the Nucleolus May Prevent Its Proper Recognition by Specific Antibodies, Eur. J. Histochem., 2005, vol. 49, pp. 117–124.
Zatsepina, O.V., Dudnic, O.A., Todorov, I.T., Thiry, M., Spring, H., and Trendelenburg, M.F., Experimental Induction of Prenucleolar Bodies (PNBs) in Interphase Cells: Interphase PNBs Show Similar Characteristics as Those Typically Observed at Telophase of Mitosis in Untreated Cells, Chromosoma, 1997, vol. 105, pp. 418–430.
Bridger, J.M., Kill, I.R., and Lichter, P., Association of pKi-67 with Satellite DNA of the Human Genome in Early G1 Cells, Chromosome Res., 1998, vol. 6, pp. 13–24.
Chan, P.K., Characterization and Cellular Localization of Nucleophosmin/B23 in HeLa Cells Treated with Selected Cytotoxic Agents (Studies of B23-Translocation Mechanism), Exp. Cell Res., 1992, vol. 203, pp. 174–181.
Itahana, K., Bhat, K.P., Jin, A., Itahana, Y., Hawke, D., Kobayashi, R., and Zhang, Y., Tumor Suppressor ARF Degrades B23, a Nucleolar Protein Involved in Ribosome Biogenesis and Cell Proliferation, Mol. Cell., 2003, vol. 12, pp. 1151–1164.
Huang, N., Negi, S., Szebeni, A., and Olson, M.O., Protein NPM3 Interacts with the Multifunctional Nucleolar Protein B23/Nucleophosmin and Inhibits Ribosome Biogenesis, J. Biol. Chem., 2005, vol. 280, pp. 5496–5502.
Schmidt, M.H., Broll, R., Bruch, H.P., and Duchrow, M., Proliferation Marker pKi-67 Affects the Cell Cycle in a Self-Regulated Manner, J. Cell. Biochem., 2002, vol. 87, pp. 334–341.
Rahmanzadeh, R., Hüttmann, G., Gerdes, J., and Scholzen, T., Chromophore-Assisted Light Inactivation of pKi-67 Leads to Inhibition of Ribosomal RNA Synthesis, Cell Prolif., 2007, vol. 40, pp. 422–430.
Cheutin, T., O’Donohue, M.F., Beorchia, A., Klein, C., Kaplan, H., and Ploton, D., Three-Dimensional Organization of pKi-67: A Comparative Fluorescence and Electron Tomography Study Using FluoroNanogold, J. Histochem. Cytochem., 2003, vol. 51, pp. 1411–1423.
Kametaka, A., Takagi, M., Hayakawa, T., Haraguchi, T., Hiraoka, Y., and Yoneda, Y., Interaction of the Chromatin Compaction-Inducing Domain (LR Domain) of Ki-67 Antigen with HP1 Proteins, Genes Cells, 2002, vol. 7, pp. 1231–1242.
Scholzen, T., Endl, E., Wohlenberg, C., van der Sar, S., Cowell, I.G., Gerdes, J., and Singh, P.B., The Ki-67 Protein Interacts with Members of the Heterochromatin Protein 1 (HP1) Family: a Potential Role in the Regulation of Higher-Order Chromatin Structure, J. Pathol., 2002, vol. 196, pp. 135–144.
Phair, R.D., Scaffidi, P., Elbi, C., Vecerova, J., Dey, A., Ozato, K., Brown, D.T., Hager, G., Bustin, M., and Misteli, T., Global Nature of Dynamic Protein-Chromatin Interactions in vivo: Three-Dimensional Genome Scanning and Dynamic Interaction Networks of Chromatin Proteins, Mol. Cell. Biol., 2004, vol. 24, pp. 6393–6402.
Chen, D. and Huang, S., Nucleolar Components Involved in Ribosome Biogenesis Cycle between the Nucleolus and Nucleoplasm in Interphase Cells, J. Cell Biol., 2001, vol. 153, pp. 169–176.
Saiwaki, T., Kotera, I., Sasaki, M., Takagi, M., and Yoneda, Y., In vivo Dynamics and Kinetics of pKi-67: Transition from a Mobile to an Immobile Form at the Onset of Anaphase, Exp. Cell Res., 2005, vol. 308, pp. 123–134.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Sheval, E.V., Dudnik, O.A., Abramchuk, S.S. et al. Perichromosomal layer proteins associate with chromosome scaffold and nuclear matrix throughout the cell cycle. Biochem. Moscow Suppl. Ser. A 3, 168–183 (2009). https://doi.org/10.1134/S199074780902010X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S199074780902010X