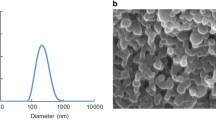
Abstract
The present work was aimed at studying the ability of three model green proteins to covalently bind to microparticles (MPs) based on poly(D,L-lactic acid) (PLA). Green fluorescent protein (sfGFP), the fusion protein of recombinant human β2-microglobulin (β2M) with sfGFP (β2M–sfGFP) and the fusion protein of recombinant human amylin (IAPP) with sfGFP (IAPP–sfGFP) were isolated using affinity chromatography. MP–PLAs were formed by the double-emulsion method. The modification of MP–PLAs by protein was confirmed by laser scanning microscopy (LSM). In addition, LSM was used to study the phagocytosis of MP–PLA modified by different proteins and free model proteins by macrophages. Recombinant sfGFP was shown to binds to the surface of particles at lower amounts compared to β2M–sfGFP and IAPP–sfGFP. This is probably due to the fact that protein amino groups that could potentially react with activated carboxyl groups on the surface of particles are sterically inaccessible for this reaction because of the sfGFP structure. The β2M and IAPP proteins, being components of the respective recombinant fusion proteins, are spacer structures between the surface of spherical particles and sfGFP. It was established that a threefold increase in the protein/particles ratio did not lead to an increase in the bound protein per unit of particle mass, which may indicate the amount of protein that can be bound per unit of particle mass is limited by the capacity of particles themselves. The study of phagocytosis of protein-modified MP–PLAs has shown that MP–PLAs containing model proteins (β2M–sfGFP and IAPP–sfGFP) on their surface are successfully phagocytized by macrophages and, thereby, can contribute to the activation of cell-mediated immune response, which is important for controlling various, including viral, infections. Phagocytosis of model proteins (β2M–sfGFP, IAPP–sfGFP) has also been shown in the present work. This may be due to the fact that both β2M and IAPP are amyloidogenic and aggregation-prone proteins. In all likelihood, the aggregates of these proteins can be absorbed by macrophages due to the increased size compared to their monomeric forms.


Similar content being viewed by others
REFERENCES
Antimonova, O.I., Grudinina, N.A., Polyakov, D.S., and Shavlovskij, M.M., Human amylin fusion protein with green fluorescent protein “Superfolder,” Estestv. Mat. Nauki Sovrem. Mire, 2016, vol. 4, no. 39, p. 15.
Begines, B., Ortiz, T., Pérez-Aranda, M., Martínez, G., Merinero, M., Argüelles-Arias, F., and Alcudia, A., Polymeric nanoparticles for drug delivery: recent developments and future prospects, Nanomaterials, 2020, vol. 10, p. 1403.
Bhattacharya, S., Naveena Lavanya Latha, J., Kumresan, R., and Singh, S., Cloning and expression of human islet amyloid polypeptide in cultured cells, Biochem. Biophys. Res. Commun., 2007, vol. 356, p. 622.
Chen, M., Rosenberg, J., Cai, X., Lee, A.C.H., Shi, J., Nguyen, M., Wignakumar, T., Mirle, V., Edobor, A.J., Fung, J., Donington, J.S., Shanmugarajah, K., Lin, Y., Chang, E., et al., Nanotraps for the containment and clearance of SARS-CoV-2, Matter, 2021, vol. 4, p. 2059.
Davies, J.Q. and Gordon, S., Isolation and culture of human macrophages, Basic Cell Culture Protocols, 2021, vol. 290, p. 105.
Fajardo-Moser, M., Berzel, S., and Moll, H., Mechanisms of dendritic cell-based vaccination against infection, Int. J. Med. Microbiol., 2008, vol. 298, p. 11.
Gamvrellis, A., Leong, D., Hanley, J.C., Xiang, S.D., Mottram, P., and Plebanski, M., Vaccines that facilitate antigen entry into dendritic cells, Immun. Cell Biol., 2004, vol. 82, p. 506.
Korzhikov-Vlakh, V., Averianov, I., Sinitsyna, E., Nashchekina, Y., Polyakov, D., Guryanov, I., Lavrentieva, A., Raddatz, L., Korzhikova-Vlakh, E., Scheper, T., and Tennikova, T., Novel pathway for efficient covalent modification of polyester materials of different design to prepare biomimetic surfaces, Polymers, 2018, vol. 10, p. 1299.
Lin, C.-Y., Lin, S.-J., Yang, Y.-C., Wang, D.-Y., Cheng, H.-F., and Yeh, M.-K., Biodegradable polymeric microsphere-based vaccines and their applications in infectious diseases, Hum. Vaccines Immunother., 2015, vol. 11, p. 650.
Pedelacq, J.-D. and Cabantous, S., Development and applications of superfolder and split fluorescent protein detection systems in biology, Int. J. Mol. Sci., 2019, vol. 20, p. 3479.
Peres, C., Matos, A.I., Conniot, J., Sainz, V., Zupančič, E., Silva, J.M., Graca, L, Gaspar, R.S., Preat, V., and Florindo, H.F., Poly(lactic acid)-based particulate systems are promising tools for immune modulation, Acta Biomater., 2017, vol. 48, p. 41.
Sakhabeev, R.G., Polyakov, D.S., Goshina, A.D., Vishnya, A.A., Kudryavtsev, I.V., Sinitsina, E.S., Korzhikov-Vlakh, V.A., Tennikova, T.B., and Shavlovsky, M.M., Enhancing the specific T cell immune response against micro- and nanoparticle immobilized antigen, Russ. J. Infect. Immun., 2021, vol. 11, no. 4, p. 777. https://doi.org/10.15789/2220-7619-ETS-1374
Simón-Vázquez, R., Peleteiro, M., and González-Fernández, Á., Polymeric nanostructure vaccines: applications and challenges, Expert Opin. Drug Delivery, 2020, vol. 17, p. 1007.
Taylor, P.C., Adams, A.C., Hufford, M.M., de la Torre, I., Winthrop, K., and Gottlieb, R.L., Neutralizing monoclonal antibodies for treatment of COVID-19, Nat. Rev. Immunol., 2021, vol. 21, p. 382.
Tyler, B., Gullotti, D., Mangraviti, A., Utsuki, T., and Brem, H., Polylactic acid (PLA) controlled delivery carriers for biomedical applications, Adv. Drug Delivery Rev., 2016, vol. 107, p. 163.
Vilos, C. and Velasquez, L.A., Therapeutic strategies based on polymeric microparticles, J. Biomed. Biotech., 2012, vol. 2012, p. 1.
Vlachopoulos, A., Karlioti, G., Balla, E., Daniilidis, V., Kalamas, T., Stefanidou, M., Bikiaris, N.D., Christodoulou, E., Koumentakou, I., Karavas, E., and Bikiaris, D.N., Poly(lactic acid)-based microparticles for drug delivery applications: an overview of recent advances, Pharmaceutics, 2022, vol. 14, p. 359.
Funding
Work on obtaining PLA-based MPs, their carboxylation, covalent immobilization of proteins on the particle surface, and characterization of the resultant polymer systems was supported by the Russian Science Foundation, project no. 21-73-20104. The works on obtaining model recombinant proteins, isolation of macrophages, and absorption of modified MPs by macrophages, as well as confocal microscopy, were carried out in the framework of state assignments from the Ministry of Science and Higher Education of the Russian Federation: SRW no. FGWG-2022-0009, registration no. NIOKTR 122020300191-9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
All procedures performed in the studies involving human beings were in agreement with the ethical standards established by the legal acts of the Russian Federation, the principles of the Basel Declaration, and the recommendations of the Local Ethics Committee of the Institute of Experimental Medicine (extract from Protocol no. 3/19 of April 25, 2019).
Additional information
Translated by E. Makeeva
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations: LSM—laser scanning microscopy; MP—microparticle; PLA—poly(D,L-lactic acid); β2M—recombinant human β2-microglobulin; IAPP—recombinant human islet amyloid polypeptide (amylin); sfGFP—green fluorescent protein superfolder; β2M–sfGFP and IAPP–sfGFP—fusion proteins of β2M and IAPP with sfGFP, respectively.
Rights and permissions
About this article
Cite this article
Sakhabeev, R.G., Polyakov, D.S., Grudinina, N.A. et al. Phagocytosis by Immune Cells of Protein-Modified Polymer Microparticles. Cell Tiss. Biol. 17, 675–681 (2023). https://doi.org/10.1134/S1990519X23060123
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X23060123