Abstract
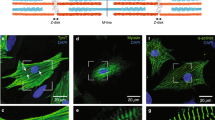
Cardiomyocytes in culture undergo reversible rearrangement of their contractile apparatus with conversion of typical myofibrils into structures resembling stress fibers of nonmuscle cells. Such rearrangement is accompanied by the replacement of cardiac actin, the main protein of myofibrils, with its smooth muscle isoform. This study shows that along with the replacement of actin isoform the key structural sarcomeric proteins are released from actin structures and stored in cell cytoplasm as inclusions not bound with actin. The data obtained are indicative of the incompatibility of smooth muscle actin with sarcomeric isoforms of these proteins and myofibrillar organization in general.


Similar content being viewed by others
REFERENCES
Antin, P.B. and Ordahl, C.P., Isolation and characterization of an avian myogenic cell line, Dev. Biol., 1991, vol. 143, pp. 111–121.
Bildjug, N.B. and Pinaev, G.P., Extracellular matrix dependence of organization of the cardiomyocyte contractile apparatus, Cell Tissue Biol., 2014, vol. 8, pp. 38–49.
Bildyug, N., Bozhokina, E., and Khaitlina, S., Contribution of A–smooth muscle actin and extracellular matrix to the in vitro reorganization of cardiomyocyte contractile system, Cell Biol. Int., 2016, vol. 40, pp. 472–477.
Borisov, A.B., Goncharova, E.I., Pinaev, G.P., and Rumiantsev, P.P., Changes in alpha-actinin localization and myofibrillogenesis in rat cardiomyocytes under cultivation, Tsitologiia, 1989, vol. 31, no. 6, pp. 642–646.
Clément, S., Chaponnier, C., and Gabbiani, G., A subpopulation of cardiomyocytes expressing A-skeletal actin is identified by a specific polyclonal antibody, Circ. Res., 1999, vol. 85, pp. e51–e58.
De La Cruz, E.M., Cofilin binding to muscle and non–muscle actin filaments: isoform-dependent cooperative interactions, J. Mol. Biol., 2005, vol. 346, pp. 557–564.
Fyrberg, E.A., Fyrberg, C.C., Biggs, J.R., Saville, D., Beall, C.J., and Ketchum, A., Functional nonequivalence of drosophila actin isoforms, Biochem. Genet., 1998, vol. 36, pp. 271–287.
Golomb, E., Ma, X., Jana, S.S., Preston, Y.A., Kawamoto, S., Shoham, N.G., Goldin, E., Conti, M.A., Sellers, J.R., and Adelstein, R.S., Identification and characterization of nonmuscle myosin II–C, a new member of the myosin II family, J. Biol. Chem., 2004, vol. 279, pp. 2800–2808.
Handel, S.E., Greaser, M.L., Schultz, E., Wang, S.M., Bulinski, J.C., Lin, J.J., and Lessard, J.L., Chicken cardiac myofibrillogenesis studied with antibodies specific for titin and the muscle and nonmuscle isoforms of actin and tropomyosin, Cell Tiss. Res., 1991, vol. 263, pp. 419–30.
Kaech, S., Fischer, M., Doll, T., and Matus, A., Isoform specificity in the relationship of actin to dendritic spines, J. Neurosci., 1997, vol. 17, pp. 9565–9572.
Khaitlina, S.Y., Functional specificity of actin isoforms, Int. Rev. Cytol., 2001, vol. 202, pp. 35–98.
Kovacs, M., Wang, F., Hu, A., Zhang, Y., and Sellers, J.R., Functional divergence of humancytoplasmic myosin II: kinetic characterization of the non-muscle IIA isoform, J. Biol. Chem., 2003, vol. 278, pp. 38132–38140.
Kumar, A., Crafword, K., Close, L., Madison, M., Lorenz, J., Doetcshman, T., Pawlowski, S., Duffy, J., Neumann, J., Robbins, J., Boivin, G.P., O’Toole, B.A., and Lessard, J.L., Rescue of cardiac A-actin-deficient mice by enteric smooth muscle-actin, Proc. Natl. Acad. Sci. U. S. A., 1997, vol. 94, pp. 4406–4411.
Martin, A.F., Phillips, R.M., Kumar, A., Crawford, K., Abbas, Z., Lessard, J.L., de Tombe, P., and Solaro, R.J., Ca(2+) activation and tension cost in myofilaments from mouse hearts ectopically expressing enteric gamma-actin, Am. J. Physiol. Heart Circ. Physiol., 2002, vol. 283, pp. H642–H649.
Mounier, N., Perriard, J.–C., Gabbiani, G., and Chaponnier, C., Transfected muscle and nonmuscle actins are differentially sorted by cultured smooth muscle and nonmuscle cells, J. Cell Sci., 1997, vol. 110, pp. 839–846.
Nag, A.C. and Cheng, M., Adult mammalian cardiac muscle cells in culture, Tissue Cell, 1981, vol. 13, pp. 515–523.
Otey, C.A. and Carpen, O., Alpha-actinin Revised: a fresh look at an old player, Cell Motil. Cytoskeleton, 2004, vol. 58, pp. 104–111.
Perrin, B.J. and Ervasti, J.M., The actin gene family: function follows isoform, Cytoskeleton Hoboken, 2010, vol. 67, pp. 630–634.
Ruzicka, D.L. and Schwartz, R.J., Sequential activation of alpha–actin genes during avian cardiogenesis: vascular smooth muscle alpha–actin gene transcripts mark the onset of cardiomyocyte differentiation, J. Cell Biol., 1988, vol. 107, pp. 2575–2586.
Schaub, M.C., Hefti, M.A., Harder, B.A., and Eppenberger, H.M., Various hypertrophic stimuli induce distinct phenotypes in cardiomyocytes, J. Mol. Med., 1997, vol. 75, pp. 901–920.
Van Bilsen, M. and Chien, K.R., Growth and hypertrophy of the heart: toward an understanding of cardiac specific and inducible gene expression, Cardiovasc. Res., 1993, vol. 27, pp. 1140–1149.
Vandekerckhove, J., Bugaisky, G., and Buckingham, M., Simultaneous expression of skeletal muscle and heart actin proteins in various striated muscle tissues and cells, J. Biol. Chem., 1986, vol. 261, pp. 1838–1843.
Von Arx, P., Bantle, S., Soldati, T., and Perriard, J.C., Dominant negative effect of cytoplasmic actin isoproteins on cardiomyocytes cytoarchitecture and function, J. Cell Biol., 1995, vol. 131, pp. 1759–1773.
Wang, F., Kovacs, M., Hu, A., Limouze, J., Harvey, E.V., and Sellers, J.R., Kinetic mechanism of non-muscle myosin IIB: functional adaptations for tension generation and maintenance, J. Biol. Chem., 2003, vol. 278, pp. 27439–27448.
Winegrad, S., Wisnewsky, C., and Schwartz, K., Effect of thyroid hormone on the accumulation of mRNA for skeletal and cardiac alpha-actin in hearts from normal and hypophysectomized rats, Proc. Natl. Acad. Sci. U. S. A., 1990, vol. 87, pp. 2456–2460.
Funding
The study was supported by the Russian Science Foundation, project no. 18-74-00129.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. We declare that all experiments with animals were carried out in compliance with generally accepted international ethical standards concerning animal welfare guaranteed by the certificate of the Institute of Cytology, Russian Academy of Sciences, identification number F18-00380 (Animal Welfare Assurance).
Additional information
Translated by I. Fridlyanskaya
Abbreviations: CM—cardiomyocyte, PBS—phosphate buffered saline.
Rights and permissions
About this article
Cite this article
Bildyug, N.B., Khaitlina, S.Y. Redistribution of Sarcomeric Myosin and α-Actinin in Cardiomyocytes in Culture upon the Rearrangement of their Contractile Apparatus. Cell Tiss. Biol. 13, 360–365 (2019). https://doi.org/10.1134/S1990519X1905002X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X1905002X