Abstract
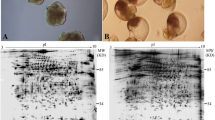
Bryozoans are typical modular organisms. They consist of repetitive structural units, the zooids. Bryozoan colonies grow by zooidal budding, with the distribution pattern of the budding loci underlying the diversity of colony forms. Budding is usually restricted to the colony periphery, where a “growing edge” or local terminal growth zones are formed. Non-budding parts of the colony can be functionally subdivided, too. In many species colonies consist of regular, often repetitive zones of feeding and non-feeding modules, associated with a periodical degeneration and regeneration of the polypide retractile tentacle crown with a gut and the accompanying musculature. The mechanisms of functional differentiation in bryozoan colonies are unknown. Presumably, budding and/or polypide recycling are induced or inhibited by certain determinants of functional specialization in different colony parts. An effective tool of their identification is the comparison of proteomes in functionally different zones. Here we report the results of proteomic analysis of three bryozoan species from the White Sea with a different colony form: Flustrellidra hispida, Terminoflustra membranaceotruncata and Securiflustra securifrons. Using differential two-dimensional electrophoresis (2D-DIGE), we compared proteomes of the growing edge, the zone with polypides and the zone without polypides. We assessed the general level of differences between the zones and revealed proteins whose relative abundance changed gradually along the proximal-distal colony axis. These proteins might be involved in the determination of the functional differentiation of the colony.
Similar content being viewed by others
Abbreviations
- LC-MS/MS:
-
liquid chromatography-tandem quadrupole time-of-flight mass spectrometry
- PAAG:
-
polyacrylamide gel
- CHAPS:
-
3-[(3-cholamidopropyl)dimethy-lammonio]-1-propane sulfonate
- ACN:
-
acetonitrile
- DTT:
-
dithiothreitol
- DIGE:
-
differential electrophoresis
- IEF:
-
iso-electric focusing
- IPG:
-
immobilized ðÍ gradient
- SDS:
-
sodium dodecyl sulfate
- Tris:
-
Tris(hydroxymethyl)aminomethane
References
Beklemishev, V.N., Osnovy sravnitel’noi anatomii bespozvonochnykh (Basics of Comparative Anatomy of Invertebrates), vol. 1: Promorfologiya (Promorphology), Moscow: Nauka, 1964.
Boardman, R.S., Cheetham, A.H., Blake, D.B., Utgaard, J., Karklins, O.L., Cook, P.L., Sandberg, P.A., Lutaud, G., and Wood, T.S., Treatise on Invertebrate Paleontology, Vol. 1: Bryozoa (Part G, revised), Lawrence: Geological Society of America, University of Kansas, Boulder, 1983, pp. 1–625.
Cheetham, A.H., Sanner, J., Taylor, P.D., and Ostrovsky, A.N., Morphological differentiation of avicularia and the proliferation of species in mid-Cretaceous Wilbertopora Cheetham, 1954 (Bryozoa: Cheilostomata), J. Paleontol., 2006, vol. 80, pp. 49–71.
Diz, A.P., Truebano, M., and Skibinski, D.O., The consequences of sample pooling in proteomics: an empirical study, Electrophoresis, 2009, vol. 30, pp. 2967–2975.
Dyrynda, P.E., A preliminary study of patterns of polypide generation-degeneration in marine cheilostome Bryozoa, in Recent and Fossil Bryozoa, Fredensborg: Olsen and Olsen, 1981, pp. 73–81.
Hageman, S.J., Complexity generated by iteration of hierarchical modules in Bryozoa, Integr. Comp. Biol., 2003, vol. 43, pp. 87–98.
Hageman, S.J., Bock, P.E., Bone, Y., and McGowran, B., Bryozoan growth habits: classification and analysis, J. Paleontol., 1998, vol. 72, pp. 418–436.
Hyman, L.H., The Invertebrates: Smaller Coelomate Groups, Vol. V: Chaetognatha, Hemi-chordata, Pogonophora, Phoronida, Ectoprocta, Brachipoda, Sipunculida, the Coelomate Bilateria, New York: McGraw-Hill, 1959.
Klyuge, G.A., Opredeliteli po faune SSSR (Identification Keys of the Fauna of USSR), vol. 76: Mshanki severnykh morey SSSR (Bryozoans of the North Seas of USSR), Moscow: Izd. Akad. Nauk SSSR, 1962.
Lidgard, S., Budding process and geometry in encrusting cheilostome bryozoans, in Bryozoa: Ordovician to Recent, Fredensborg: Olsen and Olsen, 1985, pp. 175–182.
Lidgard, S., Ontogeny in animal colonies: a persistent trend in the bryozoan fossil record, Science, 1986, vol. 232, pp. 230–232.
Lidgard, S. and Jackson, J.B., Growth in encrusting cheilostome bryozoans. I. Evolutionary trends, Paleobiology, 1989, vol. 15, pp. 255–282.
Lidgard, S., Carter, M.C., Dick, M.H., Gordon, D.P., and Ostrovsky, A.N., Division of labor and recurrent evolution of polymorphisms in a group of colonial animals, Evol. Ecol., 2012, vol. 26, pp. 233–257.
Marfenin, N.N., The concept of modular organization in development, Zh. Obscch. Biol., 1999, vol. 60, 1, pp. 6–17.
Marfenin, N.N., Fundamental patterns of modular organization in biology, Vestn. Tver. Univ. Ser. Biol. Ecol., 2008, vol. 9, pp. 147–161.
McKinney, F.K. and Jackson, J.B., Bryozoan Evolution, Boston: Unwin Hyman, 1989.
Nikulina, E.A., The evolution of colony morphogenesis in bryozoans of the order Cheilostomata, Paleontol. J., 2002, vol. 36, pp. 353–428.
Ostrovsky, A.N., Evolution of Sexual Reproduction in Marine Invertebrates: Example of Gymnolaemate Bryozoans, Dordrecht: Springer, 2013.
R Core Team, R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria, 2015. ISBN 3-900051-07-0, URL http://www.R-project.org/
Reed, C.G., Bryozoa, in Reproduction of Marine Invertebrates, Vol. 6: Echinoderms and Lophophorates. Pacific Grove: Boxwood Press, 1991, pp. 85–245.
Ryland, J.S., Bryozoans, London: Hutchinson University Library, 1970.
Ryland, J.S., Physiology and ecology of marine bryozoans, Adv. Mar. Biol., 1976, vol. 14, pp. 285–443.
Silén, L., Polymorphism, in Biology of Bryozoans, New York: Academic Press, 1977, pp. 184–232.
Stach, L.W., Observations on Carbasea indivisa Busk (Bryozoa), Proc. Zool. Soc. London, 1938, vol. 108, pp. 389–399.
Thiyagarajan, V., Wong, T., and Qian, P.Y., 2D gel-based proteome and phosphoproteome analysis during larval metamorphosis in two major marine biofouling invertebrates, J. Proteome Res., 2009, vol. 8, pp. 2708–2719.
Wang, H., Zhang, H., Wong, Y.H., Voolstra, C., Ravasi, T.B., Bajic, V., and Qian, P.Y., Rapid transcriptome and proteome profiling of a non-model marine invertebrate, Bugula neritina, Proteomics, 2010, vol. 10, pp. 2971–2981.
Wong, Y.H., Arellano, S.M., Zhang, H., Ravasi, T., and Qian, P.Y., Research dependency on de novo protein synthesis and proteomic changes during metamorphosis of the marine bryozoan Bugula neritina, Proteome Sci., 2010, vol. 8, pp. 1–14.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.A. Kutyumov, A.L. Maltseva, O.N. Kotenko, A.N. Ostrovsky, 2016, published in Tsitologiya, 2016, Vol. 58, No. 1, pp. 60–66.
The article was translated by the authors.
Rights and permissions
About this article
Cite this article
Kutyumov, V.A., Maltseva, A.L., Kotenko, O.N. et al. Functional differentiation in bryozoan colonies: a proteomic analysis. Cell Tiss. Biol. 10, 152–159 (2016). https://doi.org/10.1134/S1990519X16020073
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X16020073