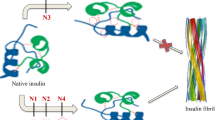
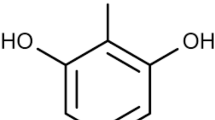
Abstract
The influence of red pigment isolated from yeast Saccharomyces cerevisiae and its low molecular weight derivate on insulin amyloid fibril formation in vitro was studied. The red pigment derivative, which presumably lacked phosphoribosyl moiety due to acid hydrolysis, retained the ability to inhibit fluorescence intensity (FI) of amyloid bound Thioflavine T. It was found that FI inhibition depended on the concentration of both pigment forms. Both forms were also able to compete with Thioflavine T for amyloid fibril binding. Electron microscopy revealed that fibrils reduced in size in the presence of red pigment.
Similar content being viewed by others
References
Adjou, K.T., Simoneau, S., Sales, N., Lamoury, F., Dormont, D., Papy-Garcia, D., Barritault, D., Deslys, J.-P., and Lasmezas, C.I., A Novel Generation of Heparan Sulfate Mimetics for the Treatment of Prion Diseases, J. Gen. Virol., 2003, vol. 84, pp. 2595–2603.
Bate, C., Salmona, M., Diomede, L., and Williams, A., Squalestatin Cures Prion-infected Neurons and Protects against Prion Neurotoxicity, J. Biol. Chem., 2004, vol. 279, pp. 14983–14990.
Bieschke, J., Russ, J., Friedrich, R.P., Ehrnhoefer, D.E., Wobst, H., Neugebauer, K., and Wanker, E.E.., EGCG Remodels Mature α-Synuclein and Amyloid-Fibrils and Reduces Cellular Toxicity, Proc. Natl. Acad. Sci. USA., 2010, vol. 107, pp. 7710–7715.
Charveriat, M., Reboul, M., Wang, Q., le, Picoli, C., Lenuzza, N., Montagnac, A., Nhiri, N., Jacquet, E., Gueritte, F., Lallemand, J.-Y., Deslys, J.-P., and Mouthon, F., New Inhibitors of Prion Replication that Target the Amyloid Precursor, J. Gen. Virol., 2009, vol. 90, pp. 1294–1301.
Chernoff, Y.O. (Ed.), Protein-Based Inheritance, Austin, Texas: Landes Bioscience, 2007.
Chernoff, Y.O., Cellular Control of Prion Formation and Propagation in Yeast, in Prions and Prion Diseases: Current Perspectives, Wymondham: Horizon Bioscience, 2004, pp. 257–303.
Chernoff, Y.O., Uptain, S.M., and Lindquist, S.L., Analysis of Prion Factors in Yeast, Meth. Enzymol., 2002, vol. 351, pp. 499–537.
Cordeiro, Y., Lima, L.M.T.R., Gomes, M.P.B., Foguel, D., and Silva, J.L., Modulation of Prion Protein Oligomerization, Aggregation, and β-Sheet Conversion by 4,4′X-Dianilino-1,1’X-Binaphthyl-5,5′X-Sulfonate (bis-ANS), J. Biol. Chem., 2004, vol. 279, pp. 5346–5352.
Demaimay, R., Chesebro, B., and Caughey, B., Inhibition of Formation of Protease-Resistant Prion Protein by Try-pan Blue, Sirius Red, Other Congo Red Analogs, Arch. Virol. Suppl., 2000, vol. 16, pp. 277–83.
Ehrnhoefer, D.E., Biesche, J., Boeddrich, A., Herbst, M., Masino, L., Lurz, R., Engemann, S., Pastore, A., and Wanker, E.E., EGCG Redirects Amyloidogenic Polypeptides into Unstructured, Off-Pathway Oligomers, Nature Struct. Mol. Biol., 2008, vol. 15, pp. 558566.
Ghaemmaghami, S., May, B.C.H., Renslo, A.R., and Prusiner, S.B., Discovery of 2-Aminothiazoles as Potent Antiprion Compounds, J. Gen. Virol., 2010, vol. 84, pp. 3408–3412.
Herczenik, E., and Gebbink, M.F.B.G., Molecular and Cellular Aspects of Protein Misfolding and Disease, FASEB J., 2008, vol. J 22, pp. 2115–2133.
Hosokawa-Muto, J., Kamatari, Y.O., Nakamura, H.K., and Kuwata, K., Variety of Antiprion Compounds Discovered through an in Silico Screen Based on Cellular-Form Prion Protein Structure: Correlation between Antiprion Activity and Binding Affinity, Antimicrob. Agents Chemother., 2009, vol. 53, pp. 765–771.
Kocisko, D.A., Baron, G.S., Rubenstein, R., Chen, J., Kuizon, S., and Caughey, B., New Inhibitors of Scrapie-Associated Prion Protein Formation in a Library of 2000 Drugs and Natural Products, J. Virol., 2003, vol. 77, pp. 10288–10294.
Korth, C., May, B.C., Cohen, F.E., and Prusiner, S.B., Acridine and Phenothiazine Derivatives as Pharmacotherapeutics for Prion Disease, Proc. Natl. Acad. Sci. USA., 2001, vol. 98, pp. 9836–9841.
Krebs, M.R.H., Bromley, E.H.C., and Donald, A.M., The Binding of Thioflavin-T to Amyloid Fibrils: Localization Implications, J. Struct. Biol., 2004, vol. 149, pp. 30–37.
Lakin, G.F., Biometriya (Biometry), Moscow: Vysshaya shkola, 1990.
Lamberto, G.R., Binolfi, A., Orcellet, M.L., Bertoncini, C.W., Zweckstetter, M., Griesinger, C., and Fernandez, C.O., Structural and Mechanistic Basis Behind the Inhibitory Interaction of PcTS on A-synuclein Amyloid Fibril Formation, Proc. Natl. Acad. Sci. USA., 2009, vol. 106, pp. 21057–21062.
Lawson, V.A., Lumicisi, B., Welton, J., Machalek, D., Gouramanis, K., Klemm, H.M., Stewart, J.D., Masters, C.L., Hoke, D.E., Collins, S.J., and Hill, A.F., Glycosaminoglycan Sulphation Affects the Seeded Misfolding of a Mutant Prion Protein, PLoS One, 2010, vol. 5, pp. e12351.
Nevzglyadova, O.V., Artemov, A.V., Mittenberg, A.G., Mikhailova, E.V., Kuznetsova, I.M., Turoverov, K.K., and Soidla, T.R., The Effect of Red Pigment on Amyloidization of Yeast Proteins, Tsitologiia, 2010, vol. 52, no. 1, pp. 80–93.
Nevzglyadova, O.V., Kuznetsova, I.M., Mikhailova, E.V., Artamonova, T.O., Artemov, A.V., Mittenberg, A.G., Kostyleva, E.I., Turoverov, K.K., Khodorkovskii, M.A., and Soidla, T.R., The Effect of red Pigment on Amyloidization of Yeast Proteins, Yeast, 2011, vol. 00, pp. 00–00.
Nevzglyadova, O.V., K, Artemov, A.V., K, Mittenberg, A.G., Mikhailova, E.V., Kuznetsova, I.M., Turoverov, K.K., and Soidla, T.R., Yeast Protein Aggregates, Containing Chaperones and Glucose Metabolism Enzymes, in Handbook of Molecular Chaperones: Roles, Structures and Mechanisms, New York: Nova Science Publ., 2010, Part 6, pp. 241–270.
Nunziante, M., Kehler, C., Maas, E., Kassack, M.U., Groschup, M., and Schatz, H.M., Charged Bipolar Suramin Derivatives Induce Aggregation of the Prion Protein at the cell Surface and Inhibit PrPSc Replication, J. Cell. Sci., 2005, vol. 118, pp. 4959–4973.
Phuan, P.-W., Zorn, J.A, Safar, J., Giles, K, Prusiner, S.B., Cohen, F.E., and May, B.C.H., Discriminating Between Cellular and Misfolded Prion Protein by Using Affinity to 9-Aminoacridine Compounds, J. Gen. Virol., 2007, vol. 88, pp. 1392–1401.
Prusiner, S.B., Shattuck Lecture-Neurodegenerative Diseases and Prions, N. Engl. J. Med., 2001, vol. 344, pp. 1516–1526.
Saupe, S.J., New Anti-prion Drugs make Yeast Blush, Trends Biotechnol., 2003, vol. 21, pp. 516–519.
Schiffer, N.W., Broadley, S.A., Hirschberger, T., Tavan, P., Kretzschmar, H.A., Giese, A., Haass, C., Hart, F.U., and Schmid, B., Identification of Anti-prion Compounds as Efficient Inhibitors of Polyglutamine Protein Aggregation in a Zebrafish Model, J Biol. Chem., 2007, vol. 282, pp. 9195–9203.
Sherman, F, Fink, GR, and Hicks, JB., Laboratory Course Manual for Methods in Yeast Genetics, Cold Spring Harbor: Cold Spring Harbor Laboratory Press, 1986.
Smirnov, M.N., Smirnov, V.N., Budowsky, E.I, Inge-Vechtomov, S.G., and Serebrjakov, N.G., Red Pigment of Adenine-Deficient Yeast Saccharomyces cerevisiae, Biochem. Biophys. Res. Commun., 1967, vol. 2, pp. 299–304.
Smirnov, M.N., The Study of the Red Pigment of the Adenine-Dependent Mutant of the Yeast Saccharomyces cerevisiae, Extended Abstract of Cand. Sci. (Biol.) Dissertation, Leningrd, 1967.
Solassol, J., Crozet, C., Perrier, V., Leclaire, J., Beranger, F., Caminade, A.-M., Meunier, B., Dormont, D., Majoral, J.-P., and Lehmann, S., Cationic Phosphorus-containing Dendrimers Reduce Prion Replication Both in Cell Culture and in Mice Infected with Scrapie, J. Gen. Virol., 2004, vol. 85, pp. 1791–1799.
Teruya, K., Kawagoe, K., Kimura, T., Chen, C.-J., Sakasegawa, Y., and Doh-ura, K., Amyloidophilic Compounds for Prion Diseases, Infectious Disorders-Drug Targets, 2009, vol. 9, pp. 15–22.
Trevitt, C.R., and Collinge, J., A Systematic Review of Prion Therapeutics in Experimental Models, Brain, 2006, vol. 129, pp. 2241–2265.
Tribouillard, D., Bach, S., Gug, F., Desban, N., Beringue, V., Andrieu, T., Dormont, D., Galons, H., Laude, H., Vilette, D., and Blondel, M., Using Budding Yeast to Screen for Antiprion Drugs, Biotechnology, 2006, vol. J 1, pp. 58–67.
Tribouillard-Tanvier, D., Beringue, V., Desban, N., Gug, F., Bach, S., Voisset, C., Galons, H., Laude, H., Vilette, D., and Blondel, M., Antihypertensive Drug Guanabenz Is Active in vivo against Both Yeast and Mammalian Prions, PLoS One, 2008, vol. 3, pp. e1981.
Uversky, V.N., Amyloidogenesis of Natively Unfolded Proteins, Curr. Alzheimer Res., 2008, vol. 5, p. 26087.
Vishnevskaya, A.B., Kushnirov, V.V., and Ter-Avanesyan, M.D., Neurodegenerative Amyloidoses: Yeast Model, Mol. Biol. (Mosc.), 2007, vol. 41, no. 2, pp. 308–315.
Wang, H., Duennwald, M.L., Roberts, B.E., Rozeboom, L.M., Zhang, Y.L., Steele, A.D., Krishnan, R., Su, L.J., Griffin, D., Mukhopadhyay, S., Hennessy, E.J., Weigele, P., Blanchard, B.J., King, J., Deniz, A.A., Buchwald, S.L., Ingram, V.M., Lindquist, S., and Shorter, J., Direct and Selective Elimination of Specific Prions and Amyloids by 4,5-Dianilinophthalimide and Analogs, Proc. Natl. Acad. Sci. USA, 2008, vol. 105, pp. 7159–7164.
Webb, S., Lekishvili, T., Loeschner, C., Sellarajah, S., Prelli, F., Wisniewski, T., Gilbert, I.H., and Brown, T.R., Mechanistic Insights into the Cure of Prion Disease by Novel Antiprion Compounds, J. Virol., 2007, vol. 81, pp. 10729–10741.
Wickner, R.B., Edskes, H.K., Shewmaker, F., Nakayashiki, T., Engel, A., McCann, L., and Kryndushkin, D., Yeast Prions: Evolution of the Prion Concept, Prion, 2007, vol. 1, pp. 94–100.
Wickner, R.B., Shewmaker, F., Edskes, H., Kryndushkin, D., Nemecek, J., McGlinchey, R., Bateman, D., and Winchester, C-L., Prion Amyloid Structure Explains Templating: How Proteins Can Be Genes, FEMS Yeast Res., 2010, vol. 10, pp. 980–991.
Wong, C., Xiong, L.W., Horiuchi, M., Raymond, L., Wehrly, K., Chesebro, B., and Caughey, B., Sulfated Glycans and Elevated Temperature Stimulate PrPSc-Dependent Cell Free Formation of Protease-resistant Prion Protein, EMBO J., 2001, vol. J 20, pp. 377–386.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mikhailova, E.V., Artemov, A.V., Snigirevskaya, E.S. et al. Effect of red pigment on insulin fibril formation in vitro. Cell Tiss. Biol. 5, 580–585 (2011). https://doi.org/10.1134/S1990519X11060095
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X11060095