Abstract
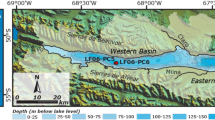
The complex study of the river water and pore solutions from the bottom sediments in the lower reaches of the Razdol’naya River was conducted in February 2010. The major ion composition of the waters indicates the submarine origin of the near-bottom and pore waters in the lower reaches of the Razdol’naya River in the winter. The river estuary extends upstream for more than 20 km. It was established that the studied sediments are reduced oozes containing pyrite, hydrotroilite, and iron monosulfide, which is direct evidence for sulfate-reduction in the sediments. The diagenesis of organic matter is the main reason for the considerable decrease in the amount of sulfates and the increase in the alkalinity of the sediment pore water. The sedimentary pore water sampled from the deep river pits is characterized by excess alkalinity that cannot be explained by sulfate-reduction and methane genesis. It was suggested that the chemical weathering of silicate minerals and the bacterial mineralization of salts of organic acids could result in the excess alkalinity of the sediment pore water.
Similar content being viewed by others
References
O. A. Alekin, Principles of Hydrogeochemistry (Gidrometizdat, Leningrad, 1970) [in Russian].
V. V. Anikiev, I. F. Barchuk, V. S. Bulkin, et al., “Fractionation of Lithophile Elements in the Bottom Sediments of the Razdol’naya River-Amur Bay Estuary,” Geokhimiya, No. 11, 1655–1661 (1988).
V. V. Anikiev, S. A. Perepelitsa, and E. N. Shumilin, “Estimate of Influence of Anthropogenic and Natural Sources on Spatial Distribution of Heavy Metals in the Bottom Sediments of Peter the Great Bay,” Geokhimiya, No. 9, 1328–1340 (1993).
S. V. Bruevich, “Determination of Alkalinity in Small Volumes of Seawater by Direct Titration,” in Guide for the Chemical Studies of Seawater (Glavsevmorput’, Leningrad, 1944) [in Russian].
M. G. Valyashko, Regularities in the Formation of Salt Deposits (Izd-vo MGU, Moscow, 1962) [in Russian].
K. A. Gomoyunov, “Hydrological Review of Amur Bay and Saifun River,” in Proceedings of Conference on the Productive Forces of Far East, Vladivostok, Soviet Union, 1927 (Vladivostok, 1927), pp. 73–91 [in Russian].
Yu. N. Gurskii and M. G. Valyashko, “Tendencies in the Formation of Chemical Composition of the Interstitial Waters of the Black Sea,” in Chemical-Oceanological Studies (Nauka, Moscow, 1977), pp. 67–84 [in Russian].
Yu. N. Gurskii, “Particular Features of the Chemical Composition of the Interstitial Waters of the White Sea,” Oceanologiya 45(2), 208–223 (2005).
O. V. Dudarev, A. I. Botsul, N. I. Savel’eva, et al., “Scales of Variability of Lithological-Biogeochemical Processes in the Razdol’naya River Estuary (Sea of Japan): Terrigenous Fluxes and Formation of Bottom Sediments,” in State of Marine Ecosystems Affected by River Run-Off (Dal’nauka, Vladivostok, 2005), pp. 4–40 [in Russian].
V. I. Zvalinskii, A. A. Mar’yash, I. V. Stonik, et al., “Production and Hydrochemical Characteristics of Ice, Subice Water, and Bottom Sediments of the Razdol’naya River Estuary (Amur Bay, Sea of Japan) during Freeze-Up,” Biol. Morya 36(3), 186–195 (2010).
I. A. Lapin, V. V. Anikeev, Yu. A. Vinnikov, et al., “Biogeochemical Aspects of the Behavior of Dissolved Organic Matter on the Estuary of the Razdol’naya River-Amur Bay of the Sea of Japan,” Okeanologiya 30(2), 234–240 (1990).
A. A. Mar’yash, N. D. Khodorenko, V. I. Zvalinskii, et al., “Chlorophyll, Humic Substances, and Organic Carbon in the Razdol’naya River Estuary during Freeze-Up,” Vestn. Dal’nevost. Otd. Ross. Akad. Nauk, No. 6, 44–51 (2010).
A. I. Polivanova, “Jet Gravity Movement as One of the Most Important Factors in the Formation of Groundwater Composition,” in Geochemistry of Natural Waters (Gidrometeoizdat, Leningrad, 1985), pp. 295–305 [in Russian].
P. P. Tishchenko, P. Ya. Tishchenko, V. I. Zvalinskii, et al., “The Carbonate System of Amur Bay (Sea of Japan) under Conditions of Hypoxia,” Oceanology 51(2), 235–246 (2011).
P. Ya. Tishchenko, G. Yu. Pavlova, E. Zyuss, et al., “The Alkali Reserve of Interstitial Water at the Sites of Methane Emission in the Sea of Okhotsk,” Geochem. Int. 39(6), 597–603 (2001).
P. Ya. Tishchenko, A. N. Derkachev, and G. Yu. Pavlova, “Formation of Carbonate Nodules at the Methane Seep Sites,” Tikhookean. Geol. 20(3), 58–67 (2001).
P. Ya. Tishchenko, C. S. Wong, G. Yu. Pavlova, et al., “The Measurement of pH Values in Seawater Using a Cell without a Liquid Junction,” Oceanology 41(6), 813–822 (2001).
P. Ya. Tishchenko, R. V. Chichkin, E. M. Il’ina, et al., “The Measurement of pH Values in Seawater Using a Cell without a Liquid Junction,” Oceanology 42(1), 27–35 (2002).
P. Ya. Tishchenko, Ch. Sh. Vong, T. I. Volkova, et al., “Carbonate System of the Razdol’naya River Estuary (Amur Bay, Sea of Japan),” Biol. Morya 31, 51–60 (2005).
P. Ya. Tishchenko, K. Val’mann, N. A. Vasilevskaya, et al., “The Contribution of Organic Matter to the Alkaline Reserve of Natural Waters,” Oceanology 46(2), 192–199 (2006).
P. Ya. Tishchenko, V. B. Lobanov, V. I. Zvalinskii, et al., “Seasonal Hypoxy of Amur Bay, Sea of Japan,” Izv. Tikhook. Inst. Rybn. Khoz. Okeanogr. 165, 108–129 (2011).
S. L. Clegg and M. Whitfield, “Activity Coefficients in Natural Waters,” in Activity Coefficients in Electrolyte Solution, Ed. by K. S. Pitzer (CRS Press, London, 1991), pp. 279–434.
R. J. Diaz and R. Rosenberg, “Spreading Dead Zones and Consequences for Marine Ecosystems,” Science 321, 926–929 (2008).
Guide To Best Practices for Ocean CO 2 Measurements, Ed. by A. G. Dickson, C. L. Sabine, and J. R. Christian, PICES Spec. Publ. 3 (2007).
S. V. Golubev, A. Bauer, and O. S. Pokrovsky, “Effect of pH and Organic Ligands on the Kinetics of Smectite Dissolution at 25°C,” Geochim. Cosmochim. Acta 70, 4436–4451 (2006).
K. Grasshoff, M. Ehrhardt, and K. Kremling, “Methods of Seawater Analysis,” 2nd ed. (Verlag Chemie, Weinheim, 1983).
A. C. Redfield, B. H. Ketchum, and F. A. Richards, “The Influence of Organisms on the Composition of Seawater,” The Seas 2, 26–77 (1963).
K. Wallmann, G. Aloisi, M. Heackel, et al., “Silicate Weathering in Anoxic Marine Sediments,” Geochim. Cosmochim. Acta 72, 3067–3090 (2008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © G.Yu. Pavlova, P.Ya. Tishchenko, N.D. Khodorenko, M.G. Shvetsova, S.G. Sagalaev, 2012, published in Tikhookeanskaya Geologiya, 2012, Vol. 31, No. 3, pp. 69–80.
Rights and permissions
About this article
Cite this article
Pavlova, G.Y., Tishchenko, P.Y., Khodorenko, N.D. et al. Major ion composition and carbonate equilibrium in the sediment pore water of the Razdol’naya River estuary of Amur Bay, the Sea of Japan. Russ. J. of Pac. Geol. 6, 251–262 (2012). https://doi.org/10.1134/S1819714012030049
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1819714012030049