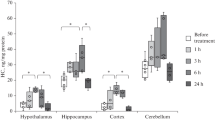
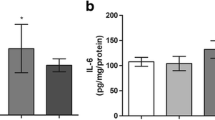
Abstract—Homocysteine is a sulfur-containing amino acid formed from methionine and considered as a risk factor for a number of pathologies. An increase in the homocysteine level (hyperhomocysteinemia, HHcy) during pregnancy leads to various complications of pregnancy, fetal hypoxia and, consequently, development of early and delayed postnatal pathologies. One of the main mechanisms of homocysteine action is induction of oxidative stress. The aim of our study was to analyze oxidative modification of proteins and activity of proteases, as well as the level of oxidative stress in the brain tissue of rats with prenatal HHcy in the first week after birth. Experimental HHcy was induced in female rats by feeding them with elevated amounts of methionine for 3 weeks before, during, and after pregnancy. In the homogenates of brain tissue of offspring with prenatal HHcy a significant increase in spontaneous protein carbonylation was observed. This result indicates a decrease in the reserve-adaptive potential of brain cells and a decrease in the resistance of the tissue to the action of free radicals. In the brains of rats with prenatal HHcy, the activity of acidic and neutral proteases was higher compared to controls which could be a result of aggregation and fragmentation of protein molecules due to carbonylation of amino acid residues. Brain tissues of newborn rats with prenatal HHcy were also characterized by a high content of hydrogen peroxide, malondialdehyde as the marker of lipid peroxidation, and a decrease in the activity of the antioxidant enzymes superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase. Thus, the data obtained indicate a significant increase in the irreversible process of oxidative modification of proteins in the brain of newborn rats with prenatal HHcy due to oxidative stress. These processes contribute to the mechanisms of homocysteine neurotoxicity during the critical period of brain development in the early postnatal period, during which intensive neurogenesis, synaptogenesis, and formation of neural networks take place.



Similar content being viewed by others
REFERENCES
Arutyunyan, A.V., Kerkeshko, G.O., Milyutina, Yu.P., Shcherbitskaya, A.D., and Zaloznyaya, I.V. Biokhimiya, 2021, vol. 86, no. 6, pp. 871–884.
Marseglia, L., D’Angelo, G., Manti, S., Arrigo, T., Barberi, I., Reiter, R.J., and Gitto, E., Oxid. Med. Cell. Longev, 2014, no. 358375.
Mal'tseva, N.V., Volchegorskii, I.A., and Shemyakov, S.E., Neurochem. J., 2017, vol. 11, pp. 50–56.
Baydas, G., Koz, S.T., Tuzcu, M., Nedzvetsky, V.S., and Etem, E., Int. J. Dev. Neurosci., 2007, vol. 25, pp. 133—139.
Koz, S.T., Gouwy, N.T., Demir, N., Nedzvetsky, V.S., Etem, E., and Baydas, G., Int. J. Dev. Neurosci., 2010, vol. 28, pp. 325–329.
Troen, A.M., Prog. Neuropsychopharm. Biol. Psychiatry, 2005, vol. 29, pp. 1140–1151.
Arutyunyan, A.V., Pustygina, A.V., Milyutina, Yu.P., Zaloznyaya, I.V., and Kozina, L.S., Molekulyarnaya Meditsina, 2015, no. 5, pp. 41–46.
Yakovleva, O., Bogatova, K., Mukhtarova, R., Yakovlev, A., Shakhmatova, V., Gerasimova, E., Ziyatdinova, G., Hermann, A., and Sitdikova, G., Biomolecules, 2020, vol. 10, no. 7, p. 995.
Yakovleva, O.V., Ziganshina, A.R., Dmitrieva, S.A., Arslanova, A.N., Yakovlev, A.V., Minibayeva, F.V., Khaertdinov, N.N., Ziyatdinova, G.K., Giniatullin, R.A., and Sitdikova, G.F., Oxid. Med. Cell. Longev., 2018, vol. 2018, no. 2, pp. 2746837—2746837.
Sharma, M., Tiwari, M., and Tiwari, R.K., Basic Clin. Pharmacol. Toxicol., 2015, vol. 117, pp. 287–296.
Herrmann, W. and Obeid, R., Clin Chem Lab Med., 2011, vol. 49, no. 3.
Gerasimova, E., Burkhanova, G., Chernova, K., Zakharov, A., Enikeev, D., Khaertdinov, N., Giniatullin, R., and Sitdikova, G., Behav. Brain Res., vol. 409, no. h. 2021, pp. 1–8.
Beard, R.S., Reynolds, J.J., and Bearden, S.E., Blood, 2011, vol. 118, no. 7, pp. 2007–2014.
Milyutina, Yu.P., Shcherbitskaya, A.D., Saltykova, E.D., Kozina, L.S., Zhuravin, I.A., Nalivaeva, N.N., and Arutyunyan A.V., Ross. Fiz. Zhurnal. im Sechenova, 2017, vol. 103, no. 11, pp. 1280–1291.
Dai, C., Fei, Y., Li, J., Shi, Y., and Yang, X., BioMed. Res. International, 2021, vol. 2021, no. 6652231.
Bolton, A.D., Phillips, M.A., and Constantine-Paton, M., J. Neurophysiol., 2013, vol. 16, no. 110, pp. 1567–1582.
Abushik, P.A., Niittykoski, M., Giniatullina, R., Shakirzyanova, A., Bart, G., and Fayuk, D., J. Neurochem., 2014, vol. 11, no. 129, pp. 264–274.
Lipton, S.A., Kim, W.K., Choi, Y.B., Kumar, S., D’Emilia, D.M., Rayuda, P.V., Arnelle, D.R., and Stampler, J.S., Proc. Natl. Acad. Sci. U.S.A., 1997, vol. 94, pp. 5923–5928.
Sibarov, D.A., Abushik, P.A., Giniatullin, R., and Antonov, S.M., Front. Cell. Neurosci., 2016, vol. 10, no. 246.
Kurmashova, E.D., Gataulina, E.D., Zefirov, A.L., Sitdikova, G.F., and Yakovlev A.V., Ross. Fiz. Zhurnal. im Sechenova, 2019, vol. 105, no. 10, pp. 1236–1246.
Arutyunyan, A.V., Milyutina, Yu.P., Shcherbitskaya, A.D., Kerkeshko, G.O., Zaloznyaya, I.V., and Mikhel’, A.V., Biokhimiya, 2020, vol. 85, no. 2, pp. 248–259.
Arutyunyan, A.V., Kozina, L.S., and Arutyunov, V.A., Zhurnal Akusherstva i Zhenskikh Boleznei, 2010, no. 59, pp. 16–23.
Pustygina, A.V., Milyutina, Y.P., Zaloznyaya, I.V., and Arutyunyan, A.V., Neurochem. J., 2015, vol. 9, pp. 60—65.
Longoni, A., Bellaver, B., Bobermin, L.D., Santos, C.L., Nonose, Y., Kolling, J., Dos, SantosT.M., de Assis, A.M., Quincozes-Santos, A., and Wyse, A.T., S, Mol. Neurobiol., 2018, vol. 55, no. 3, pp. 1966–1976.
Shcherbitskaia, A.D., Vasilev, D.S., Milyutina, Yu.P., Tumanova, N.L., Mikhel, A.V., Zalozniaia, I.V., and Arutjunyan, A.V., Cells, 2021, vol. 10, no. 6, p. 1536.
V'yushina, A.V., Pritvorova, A.V., and Flerov, M.A., Neurochem. J., 2012, vol. 6, pp. 227–232.
Cindrova-Davie, T., Herrera, E.A., Niu, Y., Kingdom, J., Giussani, D.A., and Burton, G.J., Am. J. Pathol., 2013, vol. 182, pp. 1448–1458.
Dubinina, E.E. Fiziologicheskie i kliniko-biokhimicheskie aspekty. SPb.: Meditsinskaya pressa, 2006. 400 s.
Sibrian-Vazquez, V., Escobedo, J.O., Lim, S., Samoei, G.K., and Strongin, R.M., Proc. National Academy of Sciences, 2010, vol. 107, no. 2, pp. 551–554.
Perla-Kajan, J., Twardowski, T., and Jakubowski, H., Amino Acids, 2007, vol. 12, no. 32, pp. 561–572.
Stadtman, E.R., Free Radical Res., 2006, vol. 40, p. 1250–1258.
Reinheckel, T., Noack, H., Lorenz, S., Wiswedel, I., and Augustin, W., Free Radical Res., 1998, vol. 29, pp. 297–305.
Telushkin, P.K., Problemy endokrinologi, 1998, vol. 44, no. 3, pp. 35–37.
Nystrom, T., EMBO J., 2005, vol. 24, no. 7, pp. 1311–1317.
Yakovleva, O.V., Burkhanova, G., Ziyatdinova, G., Khaertdinov, N.N., and Sitdikova, G., BioNanoScience, 2017, vol. 7, no. 1, pp. 55–158.
Parasuraman, S., Raveendran, R., and Kesavan, R., J. Pharmacology & Pharmacotherapeutics, 2010, vol. 1, no. 2, pp. 87–93.
de Oliveira, D., Souza-Silva, E., and Tonussi, C.R., Scand. J. Lab. Anim. Sci., 2009, vol. 36, no. 2, pp. 109–113.
Sliwa-Jozwik, A., Jozwik1, A., Fronczyk, W., Guszkiewicz1, A., and Kolataj, A., Animal Science Papers and Reports, 2004, vol. 22, no. 2, pp. 237–245.
Fulle, S., Di Donna, S., Puglielli, C., Pietrangelo, T., Beccafico, S., Bellomo, R., Protasi, F., and Fano, G., Exp. Gerontol., 2005, vol. 40, pp. 189–197.
Weydert, C.J. and Cullen, J.J., Nat. Protoc., 2010, vol. 5, pp. 51–66.
Brunk, U.T., Neuzil, J., and Eaton, J.W., Redox Rep., vol. 6, no. 2, pp. 91–97.
Aruoma, O., Halliwell, B., and Laughton, M.J., Biochem. J, 1989, vol. 258, no. 2, pp. 617–620.
Boldyrev, A.A. Sorosovskii Obrazovatel’nyi Zhurnal, 2001, no. 4, pp. 21–28.
Hoffman, K.B., Murray, B.A., Lynch, G., Munirathinam, S., and Bahr, B.A., Neuroscience Res., 2001, vol. 39, no. 2, pp. 167–173.
Blaise, S.A., Nedelec, E., Schroeder, H., Alberto, J.M., Bossenmeyer-Pourie, C., Gueant, J.L., and Daval, J.L., The American Journal of Pathology, 2007, vol. 170, no. 2, pp. 667–679.
Yakovlev, A.V., Kurmasheva, E.D., Giniatullin, R., Khalilov, I., and Sitdikova, G.F., Neurosci., 2017, vol. 340, pp. 153–165.
Gubskii Yu.I., Belenichev I.F., Pavlov S.V., Levitskii E.L., and Bukhtiyarova N.V., Sovremennye Problemy Toksikologii, 2006, vol. 2, pp. 37–43.
Bizzozero, O.A., in Handbook of Neurochemistry and Molecular Neurobiology, Lajtha, A., Banik, N., Ray, S.K, Eds., Boston: Springer, 2009, pp. 543–562.
Wehr, N.B. and Levine, R.L., Methods Mol. Biol., 2013, vol. 965, pp. –. 265–281.
Streck, E.L., Matte, C., Vieira, P.S., Rombaldi, F., Wannmacher, C.M.D., Wajner, M., and Wyse, A.T.S., Neurochem. Res., 2002, vol. 27, no. 12, pp. 1593–1598.
Matte, C.;., Mackedanz, V.;., Stefanello, F.M.;., Schererna, E.B.S., Andreazza, A.C., Zanotto, C., Moro, A.M., Garcia, S.C., Goncalves, C.A., Erdtmann, B., Salvador, M., and Wyse, A.T.S., Neurochem. Int., 2009, vol. 54, pp. 7–13.
Durmaz, A. and Dikmen, N., J. Enzyme Inhib. Med. Chem., 2007, vol. 22, no. 6, pp. 733–738.
Lubos, E., Loscalzo, J., and Handy, D.E., Antioxid. Redox Signal, 2007, vol. 9, pp. 1923–1940.
Il'icheva, A.S. and Fomina, M.A., Rossiiskii Mediko-Biologicheskii Vestnik im. Akademika I.P. Pavlova, 2015, vol. 1, pp. 45–51.
Fomina, M.A and Terent’ev, A.A., Rossiiskii Mediko-Biologicheskii Vestnik im. Akademika I.P. Pavlova, 2018, vol. 26, no. 2, pp. 195–212.
Shringarpure, R. and Davies, K.J.A., Free Radic. Biol. Med., 2002, vol. 32, no. 11 P, pp. 1084–1089.
Tiwari, S.C. and Soni, R.M., J. Alzheimer’s Disease & Parkinsonism, 2014, no. 4, pp. 5–9.
Durmaz, A. and Dikmen, N., J. Enzyme Inhib. Med. Chem., 2007, vol. 22, no. 6, pp. 733–738.
Dasgupta, A., Zheng, J., and Bizzozero, O.A., ASN NEURO, 2012, vol. 4, no. 3.
Zheng, J., Shanley, K.L., and Bizzozero, O.A., Neurochem. Res., 2018, vol. 43, pp. 609–618.
Mandaviya, P.R., Stolk, L., and Heil, S.G., Mol. Genet. Metab., 2014, vol. 113, pp. 243–252.
de Moreira, S.D., Figueiro, P.W., Siebert, C., Prezzi, C.A., Rohden, F., Guma, F.C.R., Manfredini, V., and Wyse, A.T.S., Neurotox. Res., 2018, vol. 33, no. 3, pp. 580–592.
ACKNOWLEDGMENTS
The work was carried out within the Strategic Academic Leadership Program of Kazan (Volga Region) Federal University and was supported the Federal Research Center of the Kazan Scien-tific Center of the Russian Academy of Sciences (D.S.A.).
Funding
The work was supported by the Russian Science Foundation project no. 20-15-00100.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare no conflict of interest.
Ethical approval. All experiments on animals were conducted in compliance with international regulations (European Communities Council Directive of November 24, 1986 (86/609/EEC)) and were approved by the local ethics committee of Kazan Federal University (protocol No. 8 of 05.05.2015).
Additional information
Corresponding author; address: Kremlevskaya ul. 18, Kazan 420008; e-mail: alv.yakovlev@gmail.com.
Rights and permissions
About this article
Cite this article
Yakovlev, A.V., Dmitrieva, S.A., Krasnova, A.N. et al. Levels of Protein Carbonylation and Activity of Proteases in the Brain of Newborn Rats with Prenatal Hyperhomocysteinemia. Neurochem. J. 16, 263–270 (2022). https://doi.org/10.1134/S181971242203014X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S181971242203014X