Abstract
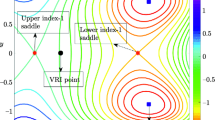
In this study, we analyze how changes in the geometry of a potential energy surface in terms of depth and flatness can affect the reaction dynamics. We formulate depth and flatness in the context of one- and two-degree-of-freedom (DOF) Hamiltonian normal form for the saddle-node bifurcation and quantify their influence on chemical reaction dynamics [1, 2]. In a recent work, García-Garrido et al. [2] illustrated how changing the well-depth of a potential energy surface (PES) can lead to a saddle-node bifurcation. They have shown how the geometry of cylindrical manifolds associated with the rank-1 saddle changes en route to the saddle-node bifurcation. Using the formulation presented here, we show how changes in the parameters of the potential energy control the depth and flatness and show their role in the quantitative measures of a chemical reaction. We quantify this role of the depth and flatness by calculating the ratio of the bottleneck width and well width, reaction probability (also known as transition fraction or population fraction), gap time (or first passage time) distribution, and directional flux through the dividing surface (DS) for small to high values of total energy. The results obtained for these quantitative measures are in agreement with the qualitative understanding of the reaction dynamics.
















Similar content being viewed by others
Change history
22 January 2021
Changes in the attributes of the ImageObject (img) tag in the HTML file
References
Borondo, F., Zembekov, A. A., and Benito, R. M., Saddle-Node Bifurcations in the LiNC/LiCN Molecular System: Classical Aspects and Quantum Manifestations, J. Chem. Phys., 1996, vol. 105, no. 12, pp. 5068–5081.
García-Garrido, V. J., Naik, Sh. and Wiggins, S., Tilting and Squeezing: Phase Space Geometry of Hamiltonian Saddle-Node Bifurcation and Its Influence on Chemical Reaction Dynamics, Internat. J. Bifur. Chaos Appl. Sci. Engrg., 2020, vol. 30, no. 4, 2030008, 35 pp.
Wales, D., Energy Landscapes: Applications to Clusters, Biomolecules and Glasses, Cambridge: Cambridge Univ. Press, 2004.
Steinfeld, J. I., Francisco, J. S., and Hase, W. L., Chemical Kinetics and Dynamics, Englewood Cliffs, N.J.: Prentice Hall, 1989.
Levine, R. D., Molecular Reaction Dynamics, Cambridge: Cambridge Univ. Press, 2009.
Agaoglou, M., Aguilar-Sanjuan, B., García-Garrido, V. J., García-Meseguer, R., González-Montoya, F., Katsanikas, M., Krajňák, V., Naik, Sh., and Wiggins, S., Chemical Reactions: A Journey into Phase Space, https://www.chemicalreactions.io (2019).
Preuss, R., Buenker, R. J., and Peyerimhoff, S. D., Theoretical Study of the Electronically Excited States of the HNSi Molecule, Chem. Phys. Lett., 1979, vol. 62, no. 1, pp. 21–25.
Bittererová, M. and Biskupič, S., Ab initio Calculation of Stationary Points on the \(\mathrm{HF}_{2}\) Potential Energy Surface, Chem. Phys. Lett., 1999, vol. 299, no. 2, pp. 145–150.
Koseki, Sh. and Gordon, M. S., Intrinsic Reaction Coordinate Calculations for Very Flat Potential Energy Surfaces: Application to Singlet Disilenylidene Isomerization, J. Phys. Chem., 1989, vol. 93, no. 1, pp. 118–125.
Nummela, J. A. and Carpenter, B. K., Nonstatistical Dynamics in Deep Potential Wells: A Quasiclassical Trajectory Study of Methyl Loss from the Acetone Radical Cation, J. Am. Chem. Soc., 2002, vol. 124, no. 29, pp. 8512–8513.
Bowman, J. M. and Suits, A. G., Roaming Reactions: The Third Way, Phys. Today, 2011, vol. 64, no. 11, pp. 33–37.
Shepler, B. C., Han, Y., and Bowman, J. M., Are Roaming and Conventional Saddle Points for \(\rm{H_{2}CO}\) and \(\rm{CH_{3}CHO}\) Dissociation to Molecular Products Isolated from Each Other?, J. Phys. Chem. Lett., 2011, vol. 2, no. 7, pp. 834–838.
Mauguiére, F. A. L., Collins, P., Kramer, Z. C., Carpenter, B. K., Ezra, G. S., Farantos, S. C., and Wiggins, S., Roaming: A Phase Space Perspective, Annu. Rev. Phys. Chem., 2017, vol. 68, no. 1, pp. 499–524.
Hare, S. R. and Tantillo, D. J., Post-Transition State Bifurcations Gain Momentum — Current State of the Field, Pure Appl. Chem., 2017, vol. 89, no. 6, pp. 679–698.
Wiggins, S., Ordinary Differential Equations, https://open.umn.edu/opentextbooks/textbooks/ordinarydifferential-equations (2017).
Uzer, T., Jaffé, Ch., Palacián, J., Yanguas, P., and Wiggins, S., The Geometry of Reaction Dynamics, Nonlinearity, 2002, vol. 15, no. 4, pp. 957–992.
Wiggins, S., The Role of Normally Hyperbolic Invariant Manifolds (NHIMs) in the Context of the Phase Space Setting for Chemical Reaction Dynamics, Regul. Chaotic Dyn., 2016, vol. 21, no. 6, pp. 621–638.
Naik, Sh. and Wiggins, S., Finding Normally Hyperbolic Invariant Manifolds in Two and Three Degrees of Freedom with Hénon – Heiles-Type Potential, Phys. Rev. E, 2019, vol. 100, no. 2, 022204, 14 pp.
de Souza, R. T., Huizenga, J. R., and Schröder, W. U., Effect of a Steep Gradient in the Potential Energy Surface on Nucleon Exchange, Phys. Rev. C, 1988, vol. 37, no. 5, pp. 1901–1919.
Doye, J. P. K. and Wales, D. J., On Potential Energy Surfaces and Relaxation to the Global Minimum, J. Chem. Phys., 1996, vol. 105, no. 18, pp. 8428–8445.
Suits, A. G., Roaming Reactions and Dynamics in the van der Waals Region, Annu. Rev. Phys. Chem., 2020, vol. 71, no. 1, pp. 77–100.
De Leon, N. and Berne, B. J., Intramolecular Rate Process: Isomerization Dynamics and the Transition to Chaos, J. Chem. Phys., 1981, vol. 75, no. 7, pp. 3495–3510.
Waalkens, H. and Wiggins, S., Direct Construction of a Dividing Surface of Minimal Flux for Multi-Degree-of-Freedom Systems That Cannot Be Recrossed, J. Phys. A, 2004, vol. 37, no. 35, pp. L435–L445.
Katsanikas, M., García-Garrido, V. J., and Wiggins, S., The Dynamical Matching Mechanism in Phase Space for Caldera-Type Potential Energy Surfaces, Chem. Phys. Lett., 2020, vol. 743, 137199, pp.
Crawford, J. D., Introduction to Bifurcation Theory, Rev. Mod. Phys., 1991, vol. 63, no. 4, pp. 991–1037.
Lyu, W., Naik, Sh., and Wiggins, S., UPOsHam: A Python Package for Computing Unstable Periodic Orbits in Two-Degree-of-Freedom Hamiltonian Systems, J. Open Source Softw., 2020, vol. 5, no. 45, pp. 1684–1689.
Ezra, G. S., Waalkens, H., and Wiggins, S., Microcanonical Rates, Gap Times, and Phase Space Dividing Surfaces, J. Chem. Phys., 2009, vol. 130, no. 16, 164118, 44 pp.
Ezra, G. S. and Wiggins, S., Sampling Phase Space Dividing Surfaces Constructed from Normally Hyperbolic Invariant Manifolds (NHIMs), J. Phys. Chem. A, 2018, vol. 122, no. 42, pp. 8354–8362.
Naik, Sh. and Ross, Sh. D., Geometry of Escaping Dynamics in Nonlinear Ship Motion, Commun. Nonlinear Sci. Numer. Simul., 2017, vol. 47, pp. 48–70.
Ross, Sh. D., BozorgMagham, A. E., Naik, Sh., and Virgin, L. N., Experimental Validation of Phase Space Conduits of Transition between Potential Wells, Phys. Rev. E, 2018, vol. 98, no. 5, 052214, 6 pp.
Marston, C. C. and De Leon, N., Reactive Islands As Essential Mediators of Unimolecular Conformational Isomerization: A Dynamical Study of 3-Phospholene, J. Chem. Phys., 1989, vol. 91, no. 6, pp. 3392–3404.
De Leon, N., Mehta, M. A., and Topper, R. Q., Cylindrical Manifolds in Phase Space As Mediators of Chemical Reaction Dynamics and Kinetics: 1. Theory, J. Chem. Phys., 1991, vol. 94, no. 12, pp. 8310–8328.
De Leon, N., Mehta, M. A., and Topper, R. Q., Cylindrical Manifolds in Phase Space As Mediators of Chemical Reaction Dynamics and Kinetics: 2. Numerical Considerations and Applications to Models with Two Degrees of Freedom, J. Chem. Phys., 1991, vol. 94, no. 12, pp. 8329–8341.
Naik, Sh. and Wiggins, S., Finding Normally Hyperbolic Invariant Manifolds in Two and Three Degrees of Freedom with Hénon – Heiles-Type Potential, Phys. Rev. E, 2019, vol. 100, no. 2, 022204, 14 pp.
Waalkens, H., Burbanks, A., and Wiggins, S., A Formula to Compute the Microcanonical Volume of Reactive Initial Conditions in Transition State Theory, J. Phys. A, 2005, vol. 38, no. 45, pp. L759–L768.
ACKNOWLEDGMENTS
The authors would like to acknowledge the London Mathematical Society (Summer Research Bursary 2019) and the School of Mathematics at the University of Bristol for supporting WL. We would like to thank the anonymous reviewer for providing critical feedback and pointing towards using steepness as an alternative to flatness.
Funding
We acknowledge the support of EPSRC Grant No. EP/P021123/1 and Office of Naval Research Grant No. N00014-01-1-0769.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
MSC2010
37J05,37J15,37J20,34C23,70H05,37G05
APPENDIX
Derivation of the Normal Form Hamiltonian for the Saddle-node Bifurcation
The following is concerned with the classical Hamiltonian saddle-node bifurcation and using it to model some phenomena of interest relevant to chemical reactions —bifurcations of NHIMs, depth and flatness of the potential energy surface.
The normal form for the one DOF Hamiltonian saddle node bifurcation is given by
The Jacobian of the vector field is
In the Hamiltonian saddle node described above the equilibria move as \(\mu\) is varied. It will be useful to fix the saddle point at the origin. To do this we introduce the following coordinate transformation: \(q=x-\sqrt{\mu},p=y\). Substituting this into (A.2) gives
The equilibria are given by
We evaluate the Jacobian at each equilibrium point to determine stability:
The depth of the potential well is controlled by the cubic term in the potential energy surface. Therefore, we introduce a parameter that allows us to vary the amplitude of this term:
The depth of the potential energy surface is determined by the difference between the potential evaluated at the saddle minus the potential evaluated at the center (minumum of the well). The potential energy function is given by
Hence, for a fixed \(\mu\) (that is, the distance apart from the two equilibria) the potential is made less deep by taking large \(\alpha\).
Derivation of the Expression for the Ratio of the Bottleneck Width and the Well Width for the Coupled two DOF System
The following expression is defined as \(A\):
Then the ratio of the bottleneck width and the well width can be written as
Rights and permissions
About this article
Cite this article
Lyu, W., Naik, S. & Wiggins, S. The Role of Depth and Flatness of a Potential Energy Surface in Chemical Reaction Dynamics. Regul. Chaot. Dyn. 25, 453–475 (2020). https://doi.org/10.1134/S1560354720050044
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1560354720050044