Abstract
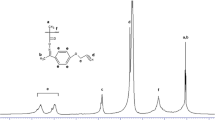
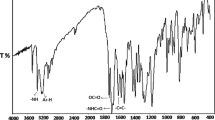
A new methacrylate monomer 4-fluorobenzyl methacrylate (FBM) was synthesized and its radical copolymerization with 2-(dimethylamino)ethyl methacrylate (DMAEMA) was studied in 1,4-dioxane solution at 65°C using 2,2′-azobisisobutyronitrile as an initiator. The synthesized monomer and copolymers were characterized by FTIR, 1H and 13C NMR spectroscopy. The analysis of reactivity ratios revealed that FBM is less reactive than DMAEMA, and copolymers formed are statistically in nature. Thermogravimetric analysis of the polymers reveals that the thermal stability of the copolymers increases with an increasing in the mole fraction of FBM in the copolymers. Glass transition temperatures of the copolymers decreased with an increasing in the mole fraction of FBM in the copolymers. The polymers exhibit the semiconducting behavior, and the electrical conductivity increases with increasing both of the temperature and DMAEMA content in copolymer.
Similar content being viewed by others
References
J. Mu, C. Zhang, J. Chen, Z. Jiang, V. V. Kireev, Polym. Sci., Ser. A 48(10), 1035 (2006).
O. A. Mel’nik, V. I. Dyachenko, L. N. Nikitin, I. V. Blagodatskikh, M. I. Buzin, G. Yu. Yurkov, Ya. S. Vygodskii, S. M. Igumnov, and V. M. Buznik, Polym. Sci., Ser. A 55(11), 625 (2011).
S. Xu and W. Liu, J. Fluorine Chem. 129, 125 (2008).
F. R. Pu, R. L. Williams, T. K. Markkula, and J. A. Hunt, Biomaterials 23, 241 (2002).
Modern Fluoropolymers: High Performance Polymers for Diverse Applications, Ed. by T. Shimizu and J. Scheirs (Wiley, Chichester, New York, Weinheim, Brisbane, Singapore, Toronto, 1997), p. 507.
Fluoropolymers, High Polymers, Ed. by L. Wall (Wiley-Interscience, New York, 1972), vol. 25.
E. E. Gilbert and B. S. Farah, US Patent No. 3544 (1970).
J. R. Griffith, R. Jacques, and G. O’Rear, US Patent No. 4,356,296 (1982).
P. E. Cassidy, J. Macromol. Sci., Rev. Macromol. Chem. Phys. C34, 1 (1994).
J. R. Griffith, ACS Div. Polym. Mater.: Sci. Eng. Proc. 50, 304 (1984).
J. R. Hurlock, US Patent No. 6025426 (2000).
V. L. Cunningham, US Patent No. 4052343 (1977).
V. L. Cunningham, US Patent No. 4129534 (1978).
C. E. Romano, Jr. and E. A. Gallo, US Patent No. 6224202 (2001).
I. Erol and O. Arslan, J. Biomater. Sci., Polym. Ed. 24, 1198 (2013).
I. Erol and C. Soykan, React. Funct. Polym. 56, 147 (2003).
S. H. Cho, M. S. Jhon, and S. H. Yuk, Eur. Polym. J. 35, 1841 (1999).
M. Fineman and S. D. Ross, J. Polym. Sci. 5, 259 (1950).
T. Kelen, and F. Tudos, J. Macromol. Sci., Chem. 9, 1 (1975).
I. F. Connerton, in Analysis of Membrane Proteins, Ed. by G. W. Gould (Portland, London, 1994), p. 177.
E. C. Z. Chan, M. J. Pelczar, and N. R. Krieg, Agar Diffusion Method, in Laboratory Exercises in Microbiology, (Mc-Graw-Hill, New York, 1993), p. 225.
J. A. Desai, U. Dayal, and P. H. Parsania, J. Macromol. Sci., Part A: Pure Appl. Chem. 33(8), 1113 (1996).
J. H. Gibbs and E. A. Di Marzio, J. Polym. Sci. 1, 1417 (1963).
T. G. Fox and P. J. Glory, J. Appl. Phys. 21, 581 (1950).
N. Abdellaoui-Arous and S. Djadoun, Macromol. Symp. 303, 123 (2011).
B. J. Holland and J. N. Hay, Polymer 42, 4825 (2001).
H. H. G. Jellinek and M. D. Luh, J. Phys. Chem. 70, 3672 (1966).
T. Ozawa, Bull. Chem. Soc. Jpn. 38, 1881 (1965).
Y. F. Yang, H. Q. Hu, Y. Li, L. S. Wan, Z. K. Xu, J. Membr. Sci. 376, 132 (2011).
C. Soykan, A. Şahan, and F. Yakuphanoglu, J. Macromol. Sci., Part A: Pure Appl. Chem. 48, 169 (2011).
J. E. Guillet and W. A. Rendall, Macromolecules 9, 224 (1986).
Y. Morishima, Y. Itoh, and S. Nozakura, Makromol. Chem. 182, 3135 (1981).
Y. Morishima, T. Kobayash, and S. Nozakura, Polym. J. 21, 267 (1989).
N. A. Weir, J. Arct, and K. Whiting, Eur. Polym. J. 26, 341 (1990).
I. Erol, J. Fluorine Chem. 129(7), 613 (2008).
A. Munoz Bonilla and M. Fernandez Garcia, Prog. Polym. Sci. 37(2), 281 (2012).
P. N. Edwards, in Organo Fluorine Chemistry, Principles and Commercial Applications, Ed. by R. E. Banks, B. E. Smart, and J. C. Tatlow (Plenum Press, New York, 1994), p. 501.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Erol, I., Arslantürk, B. & Gürler, Z. Copolymers of 4-fluoro benzyl methacrylate and 2-(dimethylamino)ethyl methacrylate: Reactivity ratios, thermal properties, biologial activity, and semi-conducting properties. Polym. Sci. Ser. B 57, 228–238 (2015). https://doi.org/10.1134/S1560090415030045
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1560090415030045