Abstract
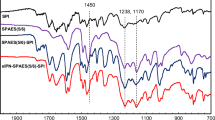
A method of obtaining new organic-inorganic nanostructured proton-exchange membranes operating via the anhydrous proton-conduction mechanism is proposed. An oligo(ethylene oxide) component serves as a proton-conducting phase in these membranes, and the sulfo derivatives of octahedral oligosilsesquioxanes of the acidic and acid-base types are used as proton-donor dopants. These compounds are synthesized via the reaction of octaaminopropyl oligosilsesquioxane with the cyclic anhydride of 2-sulfobenzoic acid at various ratios and contain sulfo groups solely or sulfo and amine groups in the organic frame. The combination of these compounds taken at concentrations of 20 and 50 wt % with α,ω-di(triethoxysilyl) oligo(oxyethylene urethane urea) and phenyltriethoxysilane via the sol-gel method gives rise to hybrid organic-inorganic proton-exchange membranes. The synthesized dopants are distributed in the oligoether component, but the nature of dopant distribution depends on their structure and concentration and has a significant impact on the structure of the resulting amorphous membranes (according to DSC, SAXS, and AFM data). The synthesized membranes are thermally stable up to 219°C. Their conductivity is provided by the segmental mobility of oligooxyethylene fragments (the Grotthuss mechanism) and, regardless of the dopant structure, is primarily determined by the number of charge carriers and the membrane structure. The temperature dependence of the conductivity is described by the Vogel-Fulcher-Tammann equation. The maximum values of the ionic conductivity are attained at 120°C under anhydrous conditions and dopant concentration of 50%: 1.03 × 10−4 for ampholytic oligosilsesquioxane and 7.43 ×10−5 S/cm for fully sulfonated oligosilsesquioxane as a dopant.
Similar content being viewed by others
References
B. P. Tripathi and V. K. Shahi, Prog. Polym. Sci. 36, 945 (2011).
Yu. A. Dobrovol’skii, E. V. Volkov, A. V. Pisareva, Yu. A. Fedotov, D. Yu. Likhachev, and A. L. Rusanov, Ross. Khim. Zh. 50(6), 95 (2006).
A. B. Yaroslavtsev, Yu. A. Dobrovol’skii, N. S. Shaglaeva, L. A. Frolova, E. V. Gerasimova, and E. A. Sanginov, Usp. Khim. 81, 191 (2012).
Y. Wang, K. S. Chen, J. Mishler, S. C. Cho, and X. C. Adroher, Appl. Energ. 88, 981 (2011).
V. V. Shevchenko, A. V. Stryutskii, and N. S. Klimenko, Theor. Exp. Chem. 47(2), 67 (2011).
L. C. Klein, Y. Daico, M. Aparicio, and F. Damay, Polymer 46, 4504 (2005).
M. Jeske, C. Soltmann, C. Ellenberg, M. Wilhelm, D. Koch, and G. Grathwohl, Fuel Cells 7, 40 (2007).
M. Michau and M. Barboiu, J. Mater. Chem. 19, 6124 (2009).
V. V. Shevchenko, N. S. Klimenko, A. V. Stryutskii, E. A. Lysenkov, and M. Ya. Vortman, Ukr. Khim. Zh. 77, 120 (2011).
V. V. Shevchenko, A. V. Stryutskii, E. A. Lysenkov, A. R. Zolotarev, and N. S. Klimenko, Ukr. Khim. Zh. 76, 58 (2010).
A. V. Stryutskii, E. A. Lysenkov, M. A. Gumennaya, A. R. Zolotarev, M. Ya. Vortman, N. S. Klimenko, and V. V. Shevchenko, Polim. Zh. 32, 383 (2010).
I. Honma, S. Nomura, and H. Nacajima, J. Membr. Sci. 185, 83 (2001).
R. Thangamuthu and C. W. Lin, J. Power Sources 150, 48 (2005).
G. Ye, C. A. Hayden, and G. R. Goward, Macromolecules 40, 1529 (2007).
J. H. Zou, Y. B. Zhao, and W. F. Shi, J. Membr. Sci. 245, 35 (2004).
S. Subianto, M. K. Mistry, N. R. Choudhury, N. K. Dutta, and R. Knott, Appl. Mater. Int. 1, 1173 (2009).
A. Mahreni, A. B. Mohamad, A. A. H. Kadhum, W. R. W. Daud, and S. E. Iyuke, J. Membr. Sci. 327, 32 (2009).
C. Hartmann-Thompson, A. Merrington, P. I. Carver, D. L. Keeley, J. L. Rousseau, D. Hucul, K. J. Bruza, L. S. Thomas, S. E. Keinath, R. M. Nowak, D. M. Katona, and P. R. Santurri, J. Appl. Polym. Sci. 110, 958 (2008).
B. Decker, C. Hartmann-Thompson, P. I. Carver, S. E. Keinath, and P. R. Santurri, Chem. Mater. 22, 942 (2010).
J. Choi, K. M. Lee, R. Wycisk, P. N. Pintauro, and P. T. Mather, J. Electrochem. Soc. B 157, 914 (2010).
V. V. Shevchenko, N. S. Klimenko, A. V. Stryutskii, E. A. Lysenkov, M. Ya. Vortman, and V. M. Rudakov, Ukr. Khim. Zh. 77, 66 (2011).
Z. Zhang, G. Liang, and T. Lu, J. Appl. Polym. Sci. 103, 2608 (2007).
D. S. Kim, H. B. Park, J. W. Rhim, and Y. M. Lee, Solid State Ionics 176, 117 (2005).
Yu. S. Lipatov, V. V. Shilov, Yu. P. Gomza, and N. E. Kruglyak, X-Ray Methods of Polymer Systems Investigation (Naukova Dumka, Kiev, 1982) [in Russian].
C. G. Vonk, J. Appl. Crystallogr. 8, 340 (1975).
G. Beaucage, J. Appl. Crystallogr. 28, 717 (1995).
L. J. Bellamy, The Infrared Spectra of Complex Molecules (Methuen, London, 1954; Inostrannaya Literatura, Moscow, 1963).
E. Pretsch, F. Bühlmann, and C. Affolter, Structure Determination of Organic Compounds: Tables of Spectral Data (Springer, 2000; Mir, Moscow, 2006).
B. D. Ghosh, K. F. Lott, and J. E. Ritchie, Chem. Mater. 18, 504 (2006).
B. D. Ghosh, K. F. Lott, and J. E. Ritchie, Chem. Mater. 17, 661 (2005).
K. F. Lott, B. D. Ghosh, and J. E. Ritchie, J. Electrochem. Soc. 153, 2044 (2006).
M. J. Park and N. P. Balsara, Macromolecules 43, 292 (2010).
J. Hou, J. Li, and L. A. Madsen, Macromolecules 43, 347 (2010).
R. Bonart, L. Morbitzer, and E. Müller, J. Macromol. Sci. B 9, 447 (1974).
A. Kyritsis, P. Pissis, and J. Grammatikakis, J. Polym. Sci., Part B: Polym. Phys. 33, 1737 (1995).
F. M. Gray, Solid Polymer Electrolytes: Fundamentals and Technological Applications (VCH, New York, 1991).
O. V. Bushkova, T. V. Sofronova, B. I. Lirova, and V. M. Zhukovskii, J. Electrochem. Soc. 41, 468 (2005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.V. Shevchenko, A.V. Stryutskii, V.N. Bliznyuk, N.S. Klimenko, A.V. Shevchuk, E.A. Lysenkov, Yu.P. Gomza, 2014, published in Russian in Vysokomolekulyarnye Soedineniya, Ser. B, 2014, Vol. 56, No. 2, pp. 202–215.
Rights and permissions
About this article
Cite this article
Shevchenko, V.V., Stryutskii, A.V., Bliznyuk, V.N. et al. Synthesis, structure, and properties of anhydrous organic-inorganic proton-exchange membranes based on sulfonated derivatives of octahedral oligosilsesquioxanes and α,ω-di(triethoxysilyl) oligo(oxyethylene urethane urea). Polym. Sci. Ser. B 56, 216–228 (2014). https://doi.org/10.1134/S1560090414020158
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1560090414020158