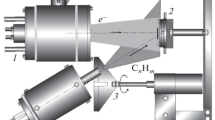
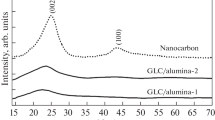
Abstract
In this work, the properties of carbon films fabricated upon the action of laser plasma on the simple gaseous hydrocarbons have been studied. The change in the properties of these films caused by subsequent thermal or laser treatment has been investigated. Carbon films deposited on a cold substrate have a complex nature, which is similar to the nature of tholins. These films contain carbon atoms in the sp2- and sp3-hybrid states in proportional amounts, as well as various structural fragments containing hydrogen and oxygen. Thermal annealing of carbon films leads to a decrease in the concentration of hydrogen and oxygen-containing structures, unification of the structure based on carbon atoms in sp3 hybridization and formation of a so-called diamond-like structure. It was shown that carbon films are formed by spherical particles with an average diameter of 7–8 nm. Laser annealing and fabrication of a film with direct constant laser exposure leads to formation of a graphene-like structure meaning sp2 hybridization of carbon.








Similar content being viewed by others
REFERENCES
Rae, J.W.B., Zhang, Yi.G., Xiaoqing, Liu., Foster, G.L., Stoll, H.M., and Whiteford, R.D.M., Atmospheric CO2 over the past 66 million years from marine archives, Ann. Rev. Earth Planet. Sci., 2021, vol. 49, pp. 609–641.
Fulcheri, L. and Schwob, Y., From methane to hydrogen, carbon black and water, Int. J. Hydrogen Energy, 1995, vol. 20, no. 3, pp. 197–202.
Global Fullerene Market—Industry Trends and Forecast to 2028. http://www.databridgemarketresearch.com/reports/global-fullerene-market.
Graphene market. http://www.fortunebusinessinsights.com/graphene-market-102930.
Sagan, C. and Khare, B., Tholins: Organic chemistry of interstellar grains and gas, Nature (London, U.K.), 1979, vol. 277, pp. 102–107.
Khare, B.N., Sagan, C., Arakawa, E.T., Suits, F., Callcott, T.A., and Williams, M.W., Optical constants of organic tholins produced in a simulated Titanian atmosphere: From soft X-ray to microwave frequencies, Icarus, 1984, vol. 60, no. 1, pp. 127–137.
Waite, J.H., Youngt, J.D.T., Cravens, T.E., Coates, A.J., Crary, F.J., Magee, B., and Westlake, J., The process of tholin formation in Titan’s upper atmosphere, Science, 2007, vol. 316, no. 5826, pp. 870–875.
Gao, G., Liu, D., Tang, Sh., Huang, C., He, M., Guo, Y., Sun, X., and Gao, B., Heat-initiated chemical functionalization of graphene, Sci. Rep., 2016, vol. 6, no. 1, p. 20034.
Díez, N., Śliwak a., Gryglewicz, S., Grzyb, B., and Gryglewicz, G., Enhanced reduction of graphene oxide by highpressure hydrothermal treatment, RSC Adv., 2015, vol. 5, pp. 81831–81837.
Tarasevich B.N., IK spektry osnovnykh klassov organicheskikh soedinenii. Spravochnye materialy (IR Spectra of the Main Classes of Organic Compounds, Reference Materials), Moscow: Mosk. Gos. Univ., 2012.
Purevsuren, B., Batbileg, S., Kuznetsova, L.I., Batkhishig, D., Namkhainorov, G., Battsetseg, M., Narangirel, G., and Kuznetsov P.N., Properties of coal from the Bayantig deposit in Mongolia and semicoking products, Solid Fuel Chem., 2019, vol. 53, no. 2, pp. 65–70.
Sivaranjini, B., Mangaiyarkarasi, R., Ganesh, V., and Umadevi, S., Vertical alignment of liquid crystals over a functionalized flexible substrate, Sci. Rep., 2018, vol. 8, p. 8891.
Dietrich, P.M., Horlacher, T., Girard-Lauriault, P-L., Gross, T., Lippitz, A., Min, H., Wirth, T., Castelli, R., Seeberger, P., and Unger, W.E.S., Multimethod chemical characterization of carbohydrate-functionalized surfaces, J. Carbohydr. Chem., 2011, p. 30, pp. 361–372.
Mitchell, S.A., Davidson, M.R., Emmison, N., and Bradley, R.H., Isopropyl alcohol plasma modification of polystyrene surfaces to influence cell attachment behaviour, Surf. Sci., 2004, vol. 561, pp. 110–120.
Arjunan, A., Balasubramanian, V., and Vaiyapuri, N., Hydrogen sorption in phosphorous substituted carbon material, Indian J. Chem., 2015, vol. 54A, pp. 1423–1433.
Ganguly, A., Sharma, S., Papakonstantinou, P., and Hamilton, J., Probing the thermal deoxygenation of graphene oxide using high-resolution in situ X-ray-based spectroscopies, J. Phys. Chem. C, 2011, vol. 115, pp. 17009–17019.
Mérel, P., Tabbal, M., Chaker, M., Moisa, S., and Margot, J., Direct evaluation of the sp 3 content in diamond-like-carbon films by XPS, Appl. Surf. Sci., 1998, vol. 136, pp. 105–110.
Murugesan, M., Nohira, H., Kobayashi, H., Fukushima, T., Tanaka, T., and Koyanagi, M., Locally induced stress in stacked ultrathin Si wafers: XPS and μ-Raman study, in Proceedings of the IEEE 62nd Electronic Components and Technology Conference, 2012, pp. 625–629.
Lomon, J., Chaiyabin, P., Saisopa, T., Seawsakul, K., Saowiang, N., Promsakha, K., Poolcharuansin, P., Pasaja, N., Chingsungnoen, A., and Supruangnet, R., XPS and XAS preliminary studies of diamond-like carbon films prepared by HiPIMS technique, J. Phys.: Conf. Ser., 2018, vol. 1144, p. 012048.
Taylor, J.A., Further examination of the Si KLL Auger line in silicon nitride thin films, Appl. Surf. Sci., 1981, vol. 7, nos. 1–2, pp. 168–184.
Dane, A., Demirok, U.K., Aydinli, A., and Suzer, S., X-ray photoelectron spectroscopic analysis of Si nanoclusters in SiO2 matrix, J. Phys. Chem. B, 2006, vol. 110, no. 3, pp. 1137–1140.
Jensen, D.S., Kanyal, S.S., Madaan, N., Vail, M.A., Dadson, A.E., Engelhard, M.H., and Linford, M.R., Silicon (100)/SiO2 by XPS, Surf. Sci. Spectra, 2013, vol. 20, no. 1, pp. 36–42.
Jensen, D.S., Kanyal, S.S., Madaan, N., Handcock, J.M., Vail, M.A., Dadson, A.E., Shutthanandan, V., Zhu, Z., Vanfleet, R., Engelhard, M.H., and Linford, M.R., Multi-instrument characterization of the surfaces and materials in microfabricated, carbon nanotube-templated thin layer chromatography plates. An analogy to ‘The blind men and the elephant’, Surf. Interface Anal., 2013, vol. 45, no. 8, pp. 1273–1282.
Gallis, S., Nikas, V., and Kaloyeros, A.E., Silicon oxycarbide thin films and nanostructures: Synthesis, properties and applications, in Modern Technologies for Creating the Thin-Film Systems and Coatings, Nikitenkov, N.N., Ed., London: IntechOpen, 2017, pp. 277–302.
Gallis, S., Nikas, V., Eisenbraun, E., Huang, M., and Kaloyeros, A.E., On the effects of thermal treatment on the composition, structure, morphology, and optical properties of hydrogenated amorphous silicon-oxycarbide, J. Mater. Res., 2009, vol. 24, no. 8, pp. 2561–2573.
Besling, W.F.A., Goossens, A., Meester, B., and Schoonman, J., Laser-induced chemical vapor deposition of nanostructured silicon carbonitride thin films, J. Appl. Phys., 1998, vol. 83, no. 1, pp. 544–553.
Smith, K.L. and Black, K.M., Characterization of the treated surfaces of silicon alloyed pyrolytic carbon and SiC, J. Vacuum Sci. Technol., A, 1984, vol. 2, no. 2, pp. 744–747.
Post, P., Wurlitzer, L., Maus-Friedrichs, W., and Weber, A.P., Characterization and applications of nanoparticles modified in-flight with silica or silica-organic coatings, Nanomaterials, 2018, vol. 8, p. 530.
Himpsel, F.J., McFeely, F.R., Taleb-Ibrahimi, A., Yarmoff, J.A., and Hollinger, G., Microscopic structure of the SiO2/Si interface, Phys. Rev. B, 1988, vol. 38, pp. 6084–6096.
Hollinger, G. and Himpsel, F.J., Probing the transition layer at the SiO2-Si interface using core level photoemission, Appl. Phys. Lett., 1984, vol. 44, pp. 93–95.
Cerofolini, G.F., Galati, C., and Renna, L., Accounting for anomalous oxidation states of silicon at the Si/SiO2 interface, Surf. Interface Anal., 2002, vol. 33, pp. 583–590.
McCafferty, E. and Wightman, J.P., Determination of the concentration of surface hydroxyl groups on metal oxide films by a quantitative XPS method, Surf. Interface Anal., 1998, vol. 26, pp. 549–564.
ACKNOWLEDGMENTS
The authors express their gratitude to the Centre for Optical and Laser Materials Research, the Centre for X-ray Diffraction Studies, Nanotechnology Interdisciplinary Centre, the Centre for Diagnostics of Functional Materials for Medicine, Pharmacology and Nanoelectronics and Centre for Physical Methods of Surface Investigation, Research Park of St.Petersurg State University.
Funding
This work was supported by the Russian Science Foundation (grant no. 22-23-20038) and the Government of St. Petersburg (Russia).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Povolotskiy, A.V., Sheremet, T.I. & Tveryanovich, Y.S. Solid Carbon Products of Isobutane Decomposition in Laser Plasma. Glass Phys Chem 48, 537–546 (2022). https://doi.org/10.1134/S1087659622600855
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659622600855