Abstract
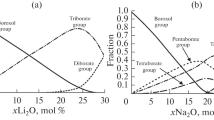
This study is aimed at the analysis of two structural models of the structure of sodium borosilicate glasses (the Dell model and the thermodynamic (TD) model), which differ significantly in their fundamental principles. Where it is possible, we compare the model’s predictions regarding the structural features of glasses in the Na2O–B2O3–SiO2 system on the near (distribution of basic structural units) and average (the content of superstructural groups as a function of glass composition) scales with the experimental data. The analysis gives an idea of the information content of both models and their correctness in terms of their predictions.














Similar content being viewed by others
REFERENCES
Wright, A.C., My borate life: An enigmatic journey, Int. J. Appl. Glass Sci., 2015, vol. 6, no. 1, pp. 45–63.
Wright, A.C., Borate structures: Crystalline and vitreous, Phys. Chem. Glasses: Eur. J. Glass Sci. Technol., Part B, 2010, vol. 51, no. 1, pp. 1–39.
Dell, W.J., Bray, P.J., and Xiao, S.Z., 11B NMR studies and structural modeling of Na2O–B2O3–SiO2 glasses of high soda content, J. Non-Cryst. Solids, 1983, vol. 58, pp. 1–16.
Feller, S., Mullenbach, T., Franke, M., Bista, S., O’Donovan-Zavada, A., Hopkins, K., Starkenberg, D., McCoy, J., Leipply, D., Stansberry, J., Troendle, E., Affatigato, M., Holland, D., Smith, M.E., Kroeker, S., Michaelis, V.K., and Wern, J.E.C., Structure and properties of barium and calcium borosilicate glasses, Phys. Chem. Glasses: Eur. J. Glass Sci. Technol., Part B, 2012, vol. 53, no. 5, pp. 210–218.
Maekawa, H., Maekawa, T., Kawamura, K., and Yokokawa, T., The structural groups of alkali silicate glasses determined from 29Si MAS-NMR, J. Non-Cryst. Solids, 1991, vol. 127, pp. 53–64.
Osipov, A.A. and Osipova, L.M., Qn distribution in silicates: Alkali silicate glasses and melts, Adv. Mater. Res., 2012, vols. 560–561, pp. 254–258.
Osipov, A.A. and Osipova, L.M., New approach to modeling of a local structure of silicate glasses and melts, J. Phys.: Conf. Ser., 2013, vol. 410, 012019.
Mysen, B.O. and Frantz, J.D., Raman spectroscopy of silicate melts at magmatic temperatures: Na2O–SiO2, K2O–SiO2 and Li2O–SiO2 binary compositions in temperature range 25–1783°C, Chem. Geol., 1992, vol. 96, pp. 321–332.
Maehara, T., Yano, T., and Shibata, S., Structural rules of phase separation in alkali silicate melts analyzed by high-temperature Raman spectroscopy, J. Non-Cryst. Solids, 2005, vol. 351, pp. 3685–3692.
Malfait, W.J., Zakaznova-Herzog, V.P., and Halter, W.E., Quantitative Raman spectroscopy: Speciation of Na-silicate glasses and melts, Am. Mineral., 2008, vol. 93, pp. 1505–1518.
Bykov, V.N., Osipov, A.A., and Anfilogov, V.N., Raman Spectroscopy of melts and glasses of the Na2O–SiO2 system, Rasplavy, 1998, no. 6, pp. 86–91.
Mysen, B.O. and Frantz, J.D., Silicate melts at magmatic temperatures: In situ structure determination to 1651°C and effect of temperature and bulk composition on the mixing behavior of structural units, Contrib. Mineral. Petrol., 1994, vol. 117, pp. 1–14.
Schneider, J., Mastelaro, V.R., Zanotto, E.D., Shakhmatrin, B.A., Vedishcheva, N.M., Wright, A.C., and Panepucci, H., Qn distribution in stoichiometric silicate glasses: Thermodynamic calculations and 29Si high resolution NMR measurements, J. Non-Cryst. Solids, 2003, vol. 325, pp. 164–178.
Shakhmatkin, B.A. and Vedishcheva, N.M., A thermodynamic approach to modeling of physical properties of oxide glasses, Glass Phys. Chem., 1998, vol. 24, no. 3, pp. 229–236.
Vedishcheva, N.M. and Wright, A.C., Chemical structure of oxide glasses: A concept for establishing structure-property relationships, in Glass Selected Properties and Crystallization, Schmelzer, J.W.P., Ed., Berlin: De Gruyter, 2014, Chap. 5, pp. 269–299.
Vedishcheva, N.M., Shakhmatkin, B.A., and Wright, A.C., Thermodynamic modelling of the structure of sodium borosilicate glasses, Phys. Chem. Glasses, 2003, vol. 44, no. 3, pp. 191–196.
Vedishcheva, N.M., Polyakova, I.G., and Wright, A.C., Short and intermediate range order in sodium borosilicate glasses: A quantitative thermodynamic approach, Phys. Chem. Glasses: Eur. J. Glass Sci. Technol., Part B, 2014, vol. 55, no. 6, pp. 225–236.
Mishra, R.K., Sudarsan, V., Kaushik, C.P., Raj, K., Kulshrehtha, S.K., and Tyagi, A.K., Effect of BaO addition on the structural aspects and thermophysical properties of sodium borosilicate glasses, J. Non-Cryst. Solids, 2007, vol. 353, pp. 1612–1617.
Nanba, T., Nishimura, M., and Miura, Y., A theoretical interpretation of the chemical shift of 29Si NMR peaks in alkali borosilicate glasses, Geochim. Cosmochim. Acta, 2004, vol. 68, no. 24, pp. 5103–5111.
Miyoshi, H., Chen, D., Masui, H., Yazawa, T., and Akai, T., Effect of calcium additive on the structural changes under heat treatment in sodium borosilicate glasses, J. Non-Cryst. Solids, 2004, vols. 345–346, pp. 99–103.
Grandjean, A., Malki, M., Montouillout, V., Debruycker, F., and Massiot, D., Electrical conductivity and 11B NMR studies of sodium borosilicate glasses, J. Non-Cryst. Solids, 2008, vol. 354, pp. 1664–1670.
Martens, R. and Müller-Warmuth, W., Structural groups and their mixing in borosilicate glasses of various compositions—an NMR study, J. Non-Cryst. Solids, 2000, vol. 265, pp. 167–175.
Winterstein-Beckmann, A., Moncke, D., Palles, D., Kamitsos, E.I., and Wondraczek, L., A Raman-spectroscopic study of indentation-induced structural changes in technical alkali-borosilicate glasses with varying silicate network connectivity, J. Non-Cryst. Solids, 2014, vol. 405, pp. 196–206.
Michel, F., Cormier, L., Lombard, P., Beuneu, B., Galoisy, L., and Calas, G., Mechanism of boron coordination change between borosilicate glasses and melts, J. Non-Cryst. Solids, 2013, vol. 379, pp. 169–176.
Fleet, M.E. and Muthupari, S., Coordination of boron in alkali borosilicate glasses using XANES, J. Non-Cryst. Solids, 1999, vol. 255, pp. 233–241.
Osipov, A.A., Osipova, L.M., and Eremyashev, V.E., Structure of alkali borosilicate glasses and melts according to Raman spectroscopy data, Glass Phys. Chem., 2013, vol. 39, no. 2, pp. 105–112.
Manara, D., Grandjean, A., and Neuville, D.R., Advances in understanding the structure of borosilicate glasses: A Raman spectroscopy study, Am. Mineral., 2009, vol. 94, pp. 777–784.
Koroleva, O.N., Shabunina, L.A., and Bykov, V.N., Structure of borosilicate glasses according to Raman spectroscopy data, Steklo Keram., 2010, no. 11, pp. 10–12.
Furukawa, T. and White, W.B., Raman spectroscopic investigation of sodium borosilicate glass structure, J. Mater. Sci., 1981, vol. 16, pp. 2689–2700.
Windisch, C.F., Jr., Pierce, E.M., Burton, S.D., and Bovaird, C.C., Deep-UV Raman spectroscopic analysis of structure and dissolution rates of silica-rich sodium borosilicate glasses, J. Non-Cryst. Solids, 2011, vol. 357, pp. 2170–2177.
Inoue, H., Masuno, A., and Watanabe, Y., Modeling of the structure of sodium borosilicate glasses using pair potentials, J. Phys. Chem. B, 2012, vol. 116, pp. 12325–12331.
Gaafar, M.S. and Marzouk, S.Y., Mechanical and structural studies on sodium borosilicate glasses doped with Er2O3 using ultrasonic velocity and FTIR spectroscopy, Phys. B (Amsterdam, Neth.), 2007, vol. 388, pp. 294–302.
Du, L.-S. and Stebbins, J.F., Nature of silicon-boron mixing in sodium borosilicate glasses: A high-resolution 11B and 17O NMR study, J. Phys. Chem. B, 2003, vol. 107, pp. 10063–10076.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Osipov, A.A., Osipova, L.M. Structure of Sodium Borosilicate Glasses: Models and Experiment. Glass Phys Chem 48, 519–536 (2022). https://doi.org/10.1134/S1087659622600521
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659622600521