Abstract
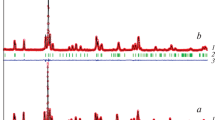
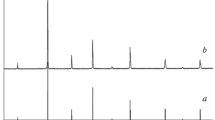
The phase relations in the subsolidus region of the GdO1.5–FeO1.5–SrO system are studied at 1200–1400°C in air. The formation of three complex perovskite-like oxides is shown: GdSr2FeO5, GdSrFeO4, and Gd2SrFe2O7, crystallizing in the tetragonal syngony. These compounds lie on the binary cross section of GdFeO3–SrO of the GdO1.5–FeO1.5–SrO system, of which the last two oxides form the homologous series GdnSrFenO3n + 1, where n = 1, 2. For the first time in conditions of the solid phase synthesis of the thermal treatment of a mixture of initial oxides of gadolinium, iron (III), and strontium carbonate at 1200°C for 5 h yielded the compound GdSr2FeO5, crystallizing in the Cs3CoCl5 (sp. gr. I4/mcm) structural type. The mechanism of its formation is determined: the rate-limiting step is the reaction of the interaction of two Gd2SrO4 and Sr3Fe2O6 oxides, crystallizing into close structural types. It is shown that ferrite GdSr2FeO5 is stable in the range of investigated temperatures of 1200 to 1400°C in air.



Similar content being viewed by others
REFERENCES
Yatoo, M.A. and Skinner, S.J., Ruddlesden-popper phase materials for solid oxide fuel cell cathodes: A short review, Mater. Today: Proc., 2022, vol. 56, no. 6, pp. 3747–3754.
Lomanova, N.A., Aurivillius phases Bim+1Fem–3Ti3O3m+3: Synthesis, structure, and properties (a review), Russ. J. Inorg. Chem., 2022, vol. 67, no. 6, pp. 741–753.
Nirala, G., Yadav, D., and Shail Upadhyay, Sh., Ruddlesden-Popper phase A2BO4 oxides: Recent studies on structure, electrical, dielectric, and optical properties, J. Adv. Ceram., 2020, vol. 9, no. 2, pp. 129–148.
Sokolova, A.N., Proskurina, O.V., Danilovich, D.P., and Gusarov, V.V., Photocatalytic properties of composites based on Y1–xBixFeO3 (0 ≤ x ≤ 0.15) nanocrystalline solid solutions with a hexagonal structure, Nanosyst. Phys. Chem. Math., 2022, vol. 13, no. 1, pp. 87–95.
Ding, P., Li, W., Zhao, H., Wu, C., Zhao, L., Dong, B., and Wang, Sh., Review on Ruddlesden-Popper perovskites as cathode for solid oxide fuel cells, J. Phys. Mater., 2021, vol. 4, no. 2, 022002.
Christopher, J. and Swamy, C.S., Studies on the catalytic decomposition of N2O on LnSrFeO4 (Ln = La, Pr, Nd, Sm, and Gd), J. Mol. Catal., 1991, vol. 68, pp. 199–213.
Nguyen, A.T., Phung, V.D., Mittova, V.O., Ngo, H.D., Vo, Th.N., le Thi, M.L., Nguyen, V.H., Mittova, I., Ya, Le, M.L.Ph., Ahn, Y.N., Kim, I.T., and Nguyen, T.L., Fabricating nanostructured HoFeO3 perovskite for lithium-ion battery anodes via co-precipitation, Scr. Mater., 2022, vol. 207, 114259.
Tikhanova, S.M., Lebedev, L.A., Martinson, K.D., Chebanenko, M.I., Buryanenko, I.V., Semenov, V.G., Nevedomskiy, V.N., and Popkov, V.I., The synthesis of novel heterojunction h-YbFeO3/o-YbFeO3 photocatalyst with enhanced fenton-like activity under visible-light, New J. Chem., 2021, vol. 45, no. 3, pp. 1541–1550.
Sheshko, T.F., Kryuchkova, T.A., Serov, Yu.M., Chislova, I.V., and Zvereva, I.A., New mixed perovskite-type Gd2–xSr1+xFe2O7 catalysts for dry reforming of methane, and production of light olefins, Catal. Ind., 2017, vol. 9, no. 2, pp. 162–169.
Singh, S. and Singh, D., Effect of increasing Sr content on structural and physical properties of K2NiF4-type phase GdSrFeO4, Ceram. Int., 2017, vol. 43, pp. 3369–3376.
Khvostova, L.V., Volkova, N.E., Gavrilova, L.Ya., Maignan, A., and Cherepanov, V.A., Gd2O3–SrO–Fe2O3 system: The phase diagram and oxygen content in oxides, Mater. Today Commun., 2021, vol. 29, p. 102885.
Volkova, N.E., Khvostova, L.V., Galaida, A.P., Gavrilova, L.Ya., and Cherepanov, V.A., Phase equilibria, crystal structure and oxygen nonstoichiometry of the complex oxides in Sm–(Sr,Ba)–(Co,Fe)–O systems, Chim. Tech. Acta, 2018, vol. 5, no. 1, pp. 55–79.
Tugova, E.A., Phase transformations in the Nd2SrAl2O7–Nd2SrFe2O7 system, Russ. J. Inorg. Chem., 2022, vol. 67, no. 6, pp. 874–880.
Fossdal, A., Einarsrud, M.-A., and Grande, T., Phase relations in the pseudo-ternary system La2O3–SrO–Fe2O3, J. Am. Ceram. Soc., 2005, vol. 88, no. 7, pp. 1988–1991.
Yang, L.T., Liang, J.K., Song, G.B., Chang, H., and Rao, G.H., Compounds and phase relations in the SrO–Fe2O3–CuO, SrO–Fe2O3–Gd2O3 and Gd2O3–Fe2O3–CuO ternary systems, J. Alloys Compd., 2003, vol. 353, pp. 301–306.
Tugova, E.A., Popova, V.F., Zvereva, I.A., and Gusarov, V.V., Diagram of LaFeO3–LaSrFeO4 system state, Glass Phys. Chem., 2006, vol. 32, no. 6, pp. 674–676.
Samaras, D. and Buisson, G., Croissance dans le flux de monocristaux de SrNdFeO4 et SrNd2Fe2O7, J. Cryst. Growth, 1976, vol. 32, no. 3, pp. 332–334.
Aksenova, T.V., Volkova, N.E., Maignan, A., and Cherepanov, V.A., Phase equilibria in the Nd2O3–BaO–Fe2O3 system: Crystal structure, oxygen content, and properties of intermediate oxides, J. Am. Ceram. Soc., 2022, vol. 105, pp. 3601–3612.
Aksenova, T.V., Elkalashy, S.I., Urusova, A.S., Cherepanov, V.A., and Vakhromeeva, A.E., Phase equilibria, crystal structure, oxygen nonstoichiometry and thermal expansion of complex oxides in the Nd2O3–SrO–Fe2O3 system, J. Solid State Chem., 2017, vol. 251, pp. 70–78.
Drofenik, M., Kolar, D., and Golič, L., Phase relations in the system SrO–Eu2O3–Fe2O3 and a new ternary phase Sr2EuFeO5, J. Less-Common Met., 1974, vol. 37, pp. 281–284.
Chislova, I.V., Matveeva, A.A., Volkova, A.V., and Zvereva, I.A., Sol-gel synthesis of nanostructured perovskite-like gadolinium ferrites, Glass Phys. Chem., 2011, vol. 37, no. 6, pp. 653–660.
Otrepina, I.V., Volodin, V.S., Zvereva, I.A., and Liu, J.-Sh., Investigation of the formation of the GdSrFeO4 oxide, Glass Phys. Chem., 2009, vol. 35, pp. 423–430.
Tugova, E.A. and Gusarov, V.V., Peculiarities of layered perovskite-related GdSrFeO4 compound solid state synthesis, J. Alloys Compd., 2011, vol. 509, no. 5, pp. 1523–1528.
Tugova, E.A., Mechanisms of the solid-state synthesis of Ln2SrFe2O7 (Ln = La, Nd, Gd, Dy) layered perovskite-related phases, Russ. J. Gen. Chem., 2019, vol. 89, no. 11, pp. 2295–2300.
Shimada, M. and Koizumi, M., Mössbauer effect of SrLnFeO4 (Ln = La, Pr, Nd, Sm, Eu, Gd), Mater. Res. Bull., 1976, vol. 11, pp. 1237–1240.
Sharma, I.B., Singh, D., and Magotra, S.K., Effect of substitution of magnetic rare earths for la on the structure, electric transport and magnetic properties of La2SrFe2O7, J. Alloys Compd., 1998, vol. 269, pp. 13–16.
Panchuk, V.V., Semenov, V.G., and Gusarov, V.V., Subsolidus phase equilibria in the GdFeO3–SrFeO3–δ system in air, Ceram. Int., 2020, vol. 46, no. 15, 24526.
Zhang, Zh., Wu, H., Meng, X., Li, J., and Zhan, Zh., Evaluation of GdSrCoO4+δ intergrowth oxides as cathode materials forintermediate-temperature solid oxide fuel cells, Electrochim. Acta, 2014, vol. 133, pp. 509–514.
Tugova, E.A., P/RS intergrowth type phases in the Ln2O3–MO–Al2O3 systems, Russ. J. Gen. Chem., 2016, vol. 86, no. 11, pp. 2410–2417.
Drofenik, M., Zupan, J., Kolar, D., and Volavšek, B., Magnetic and crystallographic investigations of some rare earth ferrite compounds, Z. Naturforsch., B, 1974, vol. 29, nos. 5–6, pp. 318–319.
Drofenik, M., Golic, L., and Kolar, D., Crystal growth of some alkaline-earth rare-earth pentaoxometallates, J. Cryst. Growth, 1979, vol. 47, pp. 739–742.
Drofenik, M. and Golič, L., Refinement of the Sr2EuFeO5 and Sr2EuAlO5 structures, Acta Crystallogr., Sect. B, 1979, vol. 35, no. 5, pp. 1059–1062.
Lim, A.R., Park, S.S., and Chang, J.-H., Structural properties of two inequivalent Cs(1) and Cs(2) sites in perovskite tricaesium pentahalogencobaltate, Cs3CoX5 (X = Cl, Br), AIP Adv., 2017, vol. 7, 105018.
Ruddlesden, S.N. and Popper, P., New compounds of the K2NiF4 type, Acta Crystallogr., 1957, vol. 10, no. 7, pp. 538–539.
Kiseleva, N.N., Stolyarenko, A.V., Ryazanov, V.V., Sen’ko, O.V., and Dokukin, A.A., Prediction of new A3+B3+C2+O4 compounds, Russ. J. Inorg. Chem., 2017, vol. 62, no. 8, pp. 1058–1066.
Barry, Th.L. and Roy, R., New rare-earth-alkaline earth oxide compounds. Predicted compound formation and new families found, J. Inorg. Nucl. Chem., 1967, vol. 29, pp. 1243–1248.
ACKNOWLEDGMENTS
The author thanks the Corresponding Member of the Russian Academy of Sciences V.V. Gusarov for supporting the research in the field of phase equilibria of refractory oxide systems.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that she has no conflicts of interest.
Rights and permissions
About this article
Cite this article
Tugova, E.A. Phase Formation in the GdFeO3–SrO System at 1200–1400°С. Glass Phys Chem 48, 614–621 (2022). https://doi.org/10.1134/S1087659622600454
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659622600454