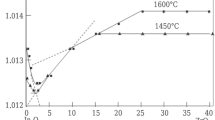
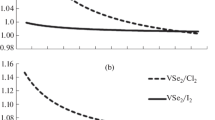
Abstract
An extensive study of the chemical mass transfer of indium oxide and In2O3−MexOy systems is performed. The efficiency of C, CO, H2, Se2, S2, In2S, In2Se, HI, HBr, HCl, InI3, InBr3, InCl3, I2, Br2 and Cl2 as transport agents for the growth of In2O3 crystals by means of the chemical vapor transport (CVT) in the sealed growth chambers is estimated. The CVT system composition and mass transfer are evaluated in the wide temperature range (627−1227°С). It is shown that among the examined substances, Cl2 is the optimal transport agent for the growth of In2O3 crystals with a minimum growth nucleus density. The compositions of MexOy−In2O3−Cl2 CVT systems were calculated for oxides (MexOy) of all non-radioactive metals of the periodic table, taking into account various types of chloride species. The effect of temperature (627−1227°С) on the total pressure and mass transfer rate of doping species (MeCln) were investigated. Doping of In2O3 by some metal oxides was predicted to be promising and some calculation results are confirmed experimentally.






Similar content being viewed by others
REFERENCES
Fornari, R., Single Crystals of Electronic Materials, London: Woodhead, 2019.
Hagleitner, D.R., Menhart, M., Jacobson, P., Blomberg, S., Schulte, K., Lundgren, E., Kubicek, M., Fleig, J., Kubel, F., Puls, C., Limbeck, A., Hutter, H., Boatner, L.A., Schmid, M., and Diebold, U., Bulk and surface characterization of In2O3(001) single crystals, Phys. Rev. B, 2012, vol. 85, 115441.
Shimada, S., Sato, O., Tsunashima, A., Kodaira, K., and Matsushita, T., Crystallization of In2O3 by vapor reaction, J. Cryst. Growth, 1987, vol. 80, pp. 366–370.
Shimada, S. and Mackenzie, K.J.D., A novel method for crystal growth of indium oxide, In2O3, from the vapour phase, J. Cryst. Growth, 1981, vol. 55, pp. 453–456.
Jozefowicz, M. and Piekarczyk, W., Preparation of In2O3 single crystals by chemical vapour transport method, Mater. Res. Bull., 1987, vol. 22, pp. 775–780.
De Wit, J.H.W., Preparation of In2O3 single-crystals via chemical transport reaction, J. Cryst. Growth, 1972, vol. 12, pp. 183–184.
Scherer, V., Janowitz, C., Krapf, A., Dwelk, H., Braun, D., and Manzke, R., Transport and angular resolved photoemission measurements of the electronic properties of In2O3 bulk single crystals, Appl. Phys. Lett., 2012, vol. 100, p. 212108.
Galazka, Z., Uecker, R., and Fornari, R., A novel crystal growth technique from the melt: Levita-tion-assisted self-seeding crystal growth method, J. Cryst. Growth, 2014, vol. 388, pp. 61–69.
Binnewies, M., Glaum, R., Schmidt, M., and Schmidt, P., Chemical Vapor Transport Reactions, Berlin: De Gruyter, 2012.
Bielz, T., Lorenz, H., Jochum, W., Kaindl, R., Klauser, F., Klotzer, B., and Penner, S., Hydrogen on In2O3: Reducibility, bonding, defect formation, and reactivity, J. Phys. Chem. C, 2010, vol. 114, pp. 9022–9029.
Wang, Ch.Y., Becker, R.W., Passow, T., et al., Photon stimulated sensor based on indium oxide nanoparticles I: Wide-concentration-range ozone monitoring in air, Sens. Actuators, B, 2011, vol. 152, pp. 235–240.
Bierwagen, O., Indium oxide – a transparent, wide-band gap semiconductor for (opto)electronic applications, Semicond. Sci. Technol., 2015, vol. 30, 024001.
Colibaba, G.V., Monaico, E.V., Goncearenco, E., Inculet, I., and Tiginyanu, I.M., Features of nano-templates manufacturing on the II-VI compound substrates, IFMBE Proc., 2016, vol. 55, pp. 188–191.
Colibaba, G.V., Sintering highly conductive ZnO:HCl ceramics by means of chemical vapor transport reactions, Ceram. Int., 2019, vol. 45, pp. 15843–15848.
Colibaba, G.V., Rusnac, D., Fedorov, V., Petrenko, P., and Monaico, E.V., Low-temperature sintering of highly conductive ZnO:Ga:Cl ceramics by means of chemical vapor transport, J. Eur. Ceram. Soc., 2021, vol. 41, pp. 443–450.
Daniels, F. and Alberty, R.A., Physical Chemistry, New York: Wiley, 1961.
Colibaba, G.V., Halide-hydrogen vapor transport for growth of ZnO single crystals with con-trollable electrical parameters, Mater. Sci. Semicond. Process., 2016, vol. 43, pp. 75–81.
Colibaba, G.V., Halide-oxide carbon vapor transport of ZnO: Novel approach for unseeded growth of single crystals with controllable growth direction, J. Phys. Chem. Solids, 2018, vol. 116, pp. 58–65.
Colibaba, G.V., Halide-carbon vapor transport of ZnO and its application perspectives for doping with multivalent metals, J. Solid State Chem., 2018, vol. 266, pp. 166–173.
Colibaba, G.V., Monaico, E.V., Goncearenco, E.P., Nedeoglo, D.D., Tiginyanu, I.M., and Nielsch, K., Growth of ZnCdS single crystals and prospects of their application as nanoporous structures, Semicond. Sci. Technol., 2014, vol. 29, 125003.
Colibaba, G.V., ZnO:HCl single crystals: Thermodynamic analysis of CVT system, feature of growth and characterization, Solid State Sci., 2016, vol. 56, pp. 1–9.
Glushko, V.P., Termodinamicheskie svoistva individual’nykh veshchestv (Thermodynamic Properties of Individual Substances), Moscow: Nauka, 1978.
Lidin, R.A., Konstanty neorganicheskikh veshchestv (Constants of Inorganic Substances), Moscow: Drofa, 2008.
Colibaba, G.V., ZnO doping efficiency by multivalent metals in complex CVT reactions, Solid State Sci., 2019, vol. 97, 105944.
Mikami, M., Sato, T., Wang, J., Masa, Y., and Isshiki, M., Improved reproducibility in zinc oxide single crystal growth using chemical vapor transport, J. Cryst. Growth, 2006, vol. 286, pp. 213–217.
Gilliland, E.R., Diffusion coefficients in gaseous systems, Ind. Eng. Chem., 1934, vol. 26, p. 681.
Glushko, V.P., Termicheskie konstanty veshchestv (Thermal Constants of Substances), Moscow: Nauka, 1982.
Chervonnyi, A.D., Thermodynamic properties of lanthanide fluorides and chlorides in the gaseous and condensed states, in Handbook on the Physics and Chemistry of Rare Earths, Amsterdam: Elsevier, 2012, vol. 42, Chap. 253.
Rabinovich, V.A. and Havin, Z.Y., Kratkii khimicheskii spravochnik (Brief Chemical Handbook), Leningrad: Khimiya, 1978.
Lidin, R.A., Khimicheskie svoistva neorganicheskikh veshchestv (Chemical Properties of Inorganic Substances), Moscow: Khimiya, 2000.
ACKNOWLEDGMENTS
The author wishes to thank Dr. E. Monaico (Technical University of Moldova) and N. Costriucova (Institute of Applied Physics, Moldova) for EDX and XRD measurement.
Funding
This work was supported by the Ministry of Education, Culture and Research of Moldova under the project no. 20.80009.5007.16 (Photosensitizers for applications in pharmaceutical medicine and photovoltaics).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that he has no conflicts of interest.
Rights and permissions
About this article
Cite this article
Colibaba, G.V. Chemical Mass Transfer of Indium Oxide and In2O3−MexOy Complex Systems. Glass Phys Chem 48, 547–557 (2022). https://doi.org/10.1134/S1087659621100448
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659621100448