Abstract
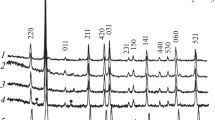
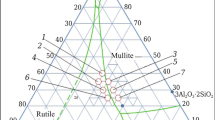
This paper presents a study of the electrically conductive properties of ceramics based on phases crystallizing in the K2O–Fe2O3–TiO2 system, when using for their synthesis the method of pyrolysis of citrate-nitrate compositions. Both single-phase titanates and their mixtures are obtained. This paper presents studies of the morphology and structure of the compounds obtained. The value of their electrical conductivity is measured in the temperature range 150–700°C. It is found that the phase with the lepidocrocite structure exhibits the highest conductivity among the considered compositions in the entire temperature range (Eact. = 0.415 eV).






Similar content being viewed by others
REFERENCES
Gorokhovsky, A.V., Tretyachenko, E.V., Goffman, V.G., Gorshkov, N.V., Fedorov, F.S., and Sevryugin, A.V., Preparation and dielectric properties of ceramics based on mixed potassium titanates with the hollandite structure, Inorg. Mater., 2016, vol. 52, no. 6, pp. 587–592.
Goffman, V.G., Gorokhovsky, A.V., Gorshkov, N.V., Fedorov, F.S., Tretychenko, E.V., and Sevrugin, A.V., Data on electrical properties of nickel modified potassium polytitanates compacted powders, Data in Brief, 2015, vol. 4, pp. 193–198.
Mumme, W.G. and Reid, A.F., Non-stoichiometric sodium iron titanate, NaxFexTi2-xO4 0.9 > x > 0.75, Acta Crystallogr., 1968, vol. 24, pp. 625–631.
Sanchez-Monjaras, T., Gorokhovsky, A., and Escalante-Garcia, J.I., Molten salt synthesis and characterization of potassium polytitanate ceramic precursors with varied TiO2/K2O molar ratios, J. Am. Ceram. Soc., 2008, vol. 91, no. 9, pp. 3058–3065.
Dion, M., Piffard, Y., and Tournoux, M., The tetratitanates M2Ti4O9 (M = Li, Na, K, Rb, Cs, Tl, Ag), J. Inorg. Nucl. Chem., 1978, vol. 40, no. 5, pp. 917–918.
Morozov, N.A., Sinelshchikova, O.Yu., Besprozvannykh, N.V., and Maslennikova, T.P., Effect of the method of synthesis on the photocatalytic and sorption properties for potassium polytitanates doped with di- and trivalent metal ions, Russ. J. Inorg. Chem., 2020, vol. 65, no. 8, pp. 1127–1134.
Mori, T., Suzuki, J., Fujimoto, K., Watanabe, M., and Hasegawa, Y., Reductive decomposition of nitrate ion to nitrogen in water on a unique hollandite photocatalyst, Appl. Catal. B: Environ., 1999, vol. 23, pp. 283–289.
Fedorov, F.S., Varezhnikov, A.S., Kiselev, I., Kolesnichenko, V.V., Burmistrov, I.N., Sommer, M., Fuchs, D., Kubel, C., Gorohovsky, A.V., and Sysoev, V.V., Potassium polytitante gas-sensing study by impendance spectroscopy, Anal. Chim. Acta, 2015, vol. 897, pp. 81–86.
Fort, A., Addabbo, T., Vignoli, V., Bertoccia, F., Magnaini, M., Atrei, A., and Gregorkiewitz, M., Gas-sensing properties and modeling of silver doped potassium hollandite, Sens. Actuators, B, 2014, vol. 194, pp. 427–439.
Cao, C., Singh, K., Hay Kan, W., Avdeev, M., and Thangadurai, V., Electrical properties of hollandite-type Ba1.33Ga2.67Ti5.33O16, K1.33Ga1.33Ti6.67O16, and K1.54Mg0.77Ti7.23O16, Inorg. Chem., 2019, vol. 58, no. 8, pp. 4782–4791.
Reddy, M.V., Sharma, N., Adams, S., Prasada Rao, R., Peterson, V.K., and Chowdari, B.V.R., Evaluation of undoped and M-doped TiO2, where M = Sn, Fe, Ni/Nb, Zr, V, and Mn, for lithium-ion battery applications prepared by the molten-salt method, RSC Adv., 2015, vol. 37, no. 5, pp. 29535–29544.
Belokoneva, E.L. and Smirnitskaya, Yu.A., Crystal structure of Na, Ti-bronze (Na0.35Fe0.10)(Ti1.54Fe0.40)(Ti1.81Fe0.19)O8, Crystallogr. Rep., 1993, vol. 38, no. 6, pp. 756–758.
Endo, T., Nagayama, H., Sato, T., and Shimada, M., Crystal growth of potassium titanates in the system K2O–Fe2O3–TiO2, J. Cryst. Growth, 1986, vol. 78, pp. 423–430.
Marimuthu, K.N., Smart, L.E., Berry, F.J., and Varadaraju, U.V., Solid state studies on K2Ti6 – xNbxFe2O16 (x = 0 and 1) and lithium insertion into K2Ti6M2O16 (M = Cr, Fe and Ga) and K2Ti5NbFe2O16 hollandite type phases, Mater. Chem. Phys., 2003, vol. 82, pp. 672–678.
Ramarkrishna, S., Mahender, N., Reddy, J.R., Kurra, S., Nagabhushan, E., and Vithal, M., Preparation and characterization of nitrogen dopper K2M2Ti6O16 (M = Cr and Fe) with enhaced photocatalytic activity, Indian J. Chem., 2015, vol. 54A, pp. 1026–1031.
Hasan, Q.U., Yang, D., Zhou, J.P., Lei, Y.X., Wang, J.Z., and Awan, S.U., Novel single-crystal hollandite K1.46Fe0.8Ti7.2O16 microrods: Synthesis, double absorption, and magnetism, Inorg. Chem., 2018, vol. 57, no. 24, pp. 15187–15197.
Bevara, S., Achary, S.N., Garg, N., Chitnis, A., Sastry, P.U., Shinde, A.B., Krishna, P.S.R., and Tyagi, A.K., Pressure and temperature dependent structural studies on hollandite type ferrotitanate and crystal structure of a high pressure phase, Inorg. Chem., 2018, vol. 57, no. 4, pp. 2157–2168.
Knyazev, A.V., Chernorukov, N.G., Letyanina, I.A., Zakharova, Y.A., and Ladenkov, I.V., Crystal structure and thermodynamic properties of dipotassium diiron(III) hexatitanium oxide, J. Therm. Anal. Calorim., 2013, vol. 112, pp. 991–996.
Hayashi, F., Furui, K., Shiiba, H., Yubuta, K., Sudare, T., Terashima, C., and Teshima, K., Flux growth of single-crystalline hollandite-type potassium ferrotitanate microrods from KCl flux, Front. Chem., 2020, vol. 20, pp. 1–7.
Machida, M., Ma, X.W., Taniguchi, H., Yabunaka, J., and Kijima, T., Pillaring and photocatalytic property of partially substituted layered titanates, Na2Ti3-xMxO7 and K2Ti4-xMxO9 (M = Mn, Fe, Co, Ni, Cu), J. Mol. Catal. A, 2000, vol. 155, pp. 131–142.
Kang, S.O., Jang, H.S., Kim, Y.I., Kim, K.B., and Jung, M.J., Study on the growth of potassium titanate nanostructures prepared by sol-gel-calcination process, Mater. Lett., 2007, vol. 61, pp. 473–477.
Groult, D., Mercey, C., and Raveau, B., Nouveaux oxydes a structure en fauillets: Les titanates de potassium non-stoechiometriques Kx(MyTi2 – y)O4, J. Solid State Chem., 1980, vol. 32, pp. 289–296.
Reid, A.F., Mumme, W.G., and Wadsley, A.D., A new class of compound \({\text{M}}_{x}^{ + }{\text{A}}_{x}^{{3 + }}{\text{T}}{{{\text{i}}}_{{2 - x}}}{{{\text{O}}}_{4}}\) (0.60 < x < 0.80) typified by RbxMnxTi2 – xO4, Acta Crystall., Sect. B, 1968, vol. 24, pp. 1228–1233.
Genkina, E.A., Maksimov, B.A., Dem’yanets, L.N., and Lazarevskaya, O.A., Synthesis and structure of a new compound of the hollandite series, Crystallogr. Rep., 1993, vol. 38, no. 6, pp. 747–748.
Sasaki, T., Watanabe, M., Fujiki, Y., Kitami, Y., and Yokoyama, M., Crystal structure of octatitanate M2Ti8O17 (M = K, Rb), J. Solid State Chem., 1991, vol. 92, pp. 537–542.
Watts, J.A., K3Ti8O17, a new alkali titanate bronze, J. Solid State Chem., 1970, vol. 1, nos. 3–4, pp. 319–325.
West, A., Solid State Chemistry and its Applications, New York: Wiley, 2014.
Irvine, J.T.S., Sinclair, D.C., and West, A.R., Electrocheramics: Characterization by impendance spectroscopy, Adv. Mater., 1990, vol. 3, pp. 132–138.
Funding
This study was carried out with the financial support of the Russian Foundation for Basic Research as part of scientific project no. 19-33-90108 “Graduate students” and with the partial support of the Ministry of Education and Science of the Russian Federation as part of a state assignment of the Institute of Silicate Chemistry, Russian Academy of Sciences (topic no. АААА-А19-119022290092-5).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Morozov, N.A., Sinelshchikova, O.Y., Besprozvannykh, N.V. et al. Citrate-Nitrate Synthesis and the Electrophysical Properties of Ceramics in the K2O–TiO2–Fe2O3 System. Glass Phys Chem 47, 481–488 (2021). https://doi.org/10.1134/S1087659621050114
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659621050114