Abstract
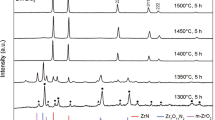
This work is devoted to the preparation of zirconium oxide nanopowders stabilized by lanthanum oxide using the method of codeposition in the presence of hydrogen peroxide. Nanopowders composed of 0.97ZrO2 · 0.03La2O3 with particles of 10–20 nm are obtained. It is found that in the temperature interval of 500–1100°C the tetragonal and monoclinic points of the zirconium oxide phase crystallize at the same time.
Similar content being viewed by others
References
Degueldre, C., Zirconia inert matrix for plutonium utilization and minor actinides disposition in reactors, J. Alloys Compd., 2007, vols. 444–445, pp. 36–41.
Vassen, R., Jarligo, M., Steinke, T., Mack, D., and Stoever, D., Overview on advanced thermal barrier coatings, Surf. Coat. Technol., 2010, vol. 205, pp. 938–942.
Sayama, K. and Arakawa, H., Photocatalytic decomposition of water and hotocatalytic reduction of carbon dioxide over zirconia catalyst, J. Phys. Chem., 1993, vol. 97, no. 3, pp. 531–533.
Gupta, T.K., Lange, F.E., and Bechtold, J.H., Mechanisms of toughening partially stabilized zirconia (PSZ), J. Am. Ceram. Soc., 1977, vol. 60, pp. 433–478.
Heuer, A.H., Transformation toughening in ZrO2-containing ceramics, J. Am. Ceram. Soc., 1987, vol. 70, pp. 689–698.
Dubok, V.A., Kabanova, M.I., Nedil’ko, S.A., and Pavlenko, N.P., Effect of synthesis method on properties of powders partially stabilized zirconia dioxide, Poroshk. Metall., 1998, no. 8, pp. 56–60.
Porter, D.L., Mechanisms of toughening partially stabilized zirconia (PSZ), J. Am. Ceram. Soc., 2006, vol. 60, pp. 183–184.
Silva, N.R.F.A., Sailer, I., Zhang, Yu., et al., Performance of zirconia for dental healthcare, Materials, 2010, vol. 3, no. 2, pp. 863–896.
Morozova, L.V., Kalinina, M.V., Panova, T.I., Arsent’ev, M.Yu., Khamova, T.V., Drozdova, I.A., and Shilova, O.A., Synthesis and study of mesoporous xerogels and nanopowders of a metastable solid solution 97ZrO2–3Y2O3 for the fabrication of catalyst substrates, Glass Phys. Chem., 2016, vol. 42, no. 3, pp. 277–283.
Khalil, K.A., Kim, S.W., and Kim, H.Y., Consolidation and mechanical properties of nanostructured hydroxyapatite-(ZrO2 + 3 mol % Y2O3) bioceramics by high-frequency induction heat sintering, Mater. Sci. Eng., 2007, vol. 456, no. 1, pp. 368–372.
Tomaev, V.V., Tveryanovich, Yu.S., and Balmakov, M.D., Control of the phase composition of nanostructured silver iodide, Nanotechnol. Russ., 2015, vol. 10, nos. 3–4, pp. 242–246.
Polikanova, A.S., Synthesis of nanosized oxydes of zirconium and yttrium by peroxo compounds pyrolysis, Cand. Sci. (Chem.) Dissertation, Moscow, 2007.
Piquemal, J.-Y., Briot, E., and Bregeault, J.-M., Preparation of materials in the presence of hydrogen peroxide: from discrete or “zero-dimensional” objects to bulk materials, Dalton Trans., 2013, vol. 42, pp. 29–45.
Osipova, V.A., Zakharova, G.S., Andreikov, E.I., Yatluk, Yu.G., and Puzyrev, I.S., Sol-gel synthesis of titanum dioxide by hydrolysis of titanium glycerolates and peroxides, Glass Phys. Chem., 2013, vol. 39, no. 4, pp. 398–402.
Kharlanov, A.N., Turakulova, A.O., Lunina, E.V., Murav’eva, G.P., and Lunin, V.V., Thermal transformations in ZrO2 doped by ittrium and lanthanum oxides, Vestn. Mosk. Univ., Ser. Khim., 1998, vol. 39, no. 3, pp. 162–165.
Yanga, Ch.-L., Hsianga, H.-I., and Chenb, Ch.-Ch., Characteristics of yttria stabilized tetragonal zirconia powder used in optical fiber connector ferrule, Ceram. Int., 2005, vol. 31, pp. 297–303.
Chevalier, J. et al., The tetragonal-monoclinic transformation in zirconia: lessons learned and future trends, J. Am. Ceram. Soc., 2009, vol. 92, no. 9, pp. 1901–1920.
Kravchik, K.V., Gomza, Yu.P., Pashkova, O.V., Belous, A.G., and Nesin, S.D., Effect of zirconium and yttrium hydroxide precipitation conditions on the fractal structure of the resulting xerogels and 0.97ZrO2 · 0.03Y2O3 powders, Inorg. Mater., 2007, vol. 43, no. 3, pp. 258–263.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © S.I. Niftaliev, I.V. Kuznetsova, M.V. Chislov, L.V. Lygina, I.A. Zvereva, 2017, published in Fizika i Khimiya Stekla.
Rights and permissions
About this article
Cite this article
Niftaliev, S.I., Kuznetsova, I.V., Chislov, M.V. et al. Synthesis and research of nanopowders composed of 0.97ZrO2 · 0.03La2O3 . Glass Phys Chem 43, 363–367 (2017). https://doi.org/10.1134/S1087659617040083
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659617040083