Abstract
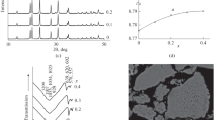
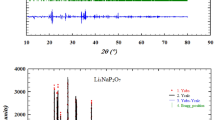
Electric properties of diphosphates Na2ZnP2O7, K2ZnP2O7, K2Zn3(P2O7)2, Li12Zn4(P2O7)5, Li2x Zn2–x P2O7 (0.30 < x ≤ 0.56), Li2ZnP2O7, and Li4P2O7 with one alkaline cation; LiNaZnP2O7, LiKZnP2O7, Na2–x K x ZnP2O7 (0.0 < x ≤ 0.6), Na x K2–x ZnP2O7 (0 < x ≤ 0.54), and Na1–x K1 + x ZnP2O7 (0 ≤ x ≤ 0.1) with two alkaline cations; and solid solutions LiNa1–x K x ZnP2O7 (0 ≤ x ≤ 1) containing three alkaline cations have been studied in system Zn2P2O7–Li2ZnP2O7–Na2ZnP2O7–K2ZnP2O7. It has been demonstrated that the nature of the alkaline cation and the crystal structure affect the electroconductivity of mixed alkali-zinc diphosphates.
Similar content being viewed by others
References
Shekhtman, G.Sh., Burmakin, E.I., and Korovenkova, E.S., Lithium-cationic conduction in the Li4P2O7–Zn2P2O7 system, Russ. J. Electrochem., 1997, vol. 33, no. 5, pp. 552–556.
Burmakin, E.I., Shekhtman, G.Sh., Aparina, E.R., and Korovenkova, E.S., Electrical conductivity of lithium pyrophosphate, Elektrokhimiya, 1992, vol. 28, no. 8, pp. 1240–1242.
Petrova, M.A., Mikirticheva, G.A., and Grebenshchikov, R.G., Phase equilibria in the Zn2P2O7–M2ZnP2O7 and ZnP2O7–ZnP2O7 (M, M′, M″ = Li, Na, K) glass-forming systems, Neorg. Mater., 2007, vol. 43, no. 9, pp. 1141–1148.
Petrova, M.A., Mikirticheva, G.A., and Grebenshchikov, R.G., Phase equilibria in the Zn2P2O7–M2ZnP2O7 and ZnP2O7–ZnP2O7 (M, M′, M″ = Li, Na, K) glass-forming systems, Inorg. Mater., 2007, vol. 43, no. 9, pp. 1024–1031.
Lapshin, A.E., Petrova, M.A., Osipova, Yu.N., Novikova, A.S., and Shepelev, Yu.F., Thermal behavior of alkali zinc phosphates, Fiz. Khim. Stekla, 2005, vol. 31, no. 5, pp. 940–948.
Lapshin, A.E., Petrova, M.A., Osipova, Yu.N., Novikova, A.S., and Shepelev, Yu.F., Thermal behavior of alkali zinc phosphates, Glass Phys. Chem., 2005, vol. 31, no. 5, pp. 684–689.
Lapshin, A.E. and Petrova, M.A., Mixed alkali-zinc diphosphates: Synthesis, structure, and properties, Fiz. Khim. Stekla, 2012, vol. 38, no. 6, pp. 718–732.
Lapshin, A.E. and Petrova, M.A., Mixed alkali-zinc diphosphates: Synthesis, structure, and properties, Glass Phys. Chem., 2012, vol. 38, no. 6, pp. 491–503.
Bokov, A.A. and Zuo-Guang, Ye., Dielectrical relaxation in relaxor ferroelectrics, J. Adv. Dielectr., 2012, vol. 2, no. 2, pp. 1241010-1–1241010-24.
Petrova, M.A., Volkov, S.N., and Bubnova, R.S., New solid solutions of mixed alkali-zinc diphosphates LiNa1–x K x ZnP2O7, Fiz. Khim. Stekla, 2014, vol. 40, no. 4, pp. 592–598.
Petrova, M.A., Volkov, S.N., and Bubnova, R.S., New solid solutions of mixed alkali-zinc diphosphates LiNa1–x K x ZnP2O7, Glass Phys. Chem., 2014, vol. 40, no. 4, pp. 447–452.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © M.A. Petrova, S.A. Petrov, O.Yu. Sinel’shchikova, V.F. Popova, V.L. Ugolkov, 2015, published in Fizika i Khimiya Stekla.
Rights and permissions
About this article
Cite this article
Petrova, M.A., Petrov, S.A., Sinel’shchikova, O.Y. et al. Electroconductivity of alkali-zinc diphosphates in partial sections of the Zn2P2O7–Li2ZnP2O7–Na2ZnP2O7–K2ZnP2O7 system. Glass Phys Chem 41, 528–532 (2015). https://doi.org/10.1134/S1087659615050132
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659615050132