Abstract
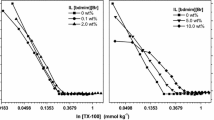
The aggregate stability of the sol of Aerosil OX-50 in solutions of cationic surfactants (SAS) of cetyldimethylethylammonium bromide (CDEAB) and cetyltrimethylammonium bromide (CTAB), as well as nonionic SAS alkylphenyl ethers of ethylene glycol, has been studied. Destabilization of the sol in the solutions of SAS was considered in the terms of the balance of surface forces and with regard to the hydrophobic effects.
Similar content being viewed by others
References
Golikova, E.V., Ioganson, O.M., and Grigor’ev, V.S., Destabilization of the sol aerosil OX-50 in solutions of surfactants: I. Aerosil OX-50 in solutions of sodium dodecyl sulfate, Glass Phys. Chem., 2013, vol. 39, no. 2 (in press).
Israelachvili, J.M., Pashley, R.M., Perer, E., and Tandon, R.K., Forces between hydrophobic surfaces in aqueous electrolyte and surfactant solutions containing common air-borne impurities, Colloids Surf., 1981, vol. 2, no. 3, pp. 287–291.
Israelachvili, J.N. and Christenson, H.K., Liquid structure and the short-range forces between surfaces in liquids, Physica A (Amsterdam), 1986, vol. 140, nos. 1–2, pp. 278–284.
Israelachvili, J. and Pashley, R., The hydrophobic interaction is long-range, decaying exponentially with distance, Nature (London), 1982, vol. 300, no. 5890, pp. 341–342.
Israelachvili, J. and Pashley, R., Measurement of hydrophobic interaction between two hydrophobic surface in aqueous electrolyte solutions, J. Colloid Interface Sci., 1984, vol. 98, no. 2, pp. 500–514.
Claesson, P.M. and Cristenson, H.K., Very long attractive forces between uncharged hydrocarbon and fluorocarbon surfaces in water, J. Phys. Chem., 1988, vol. 92, no. 6, pp. 1650–1655.
Rabinovich, Ya.I., Deryagin, B.V., Rabinovich, Ya.I., Deryagin, B.V., and Churaev, N.V., Direct measurements of the forces of attraction hydrophobic silica fibers in solutions of KCl, Kolloidn. Zh., 1987, vol. 49, no. 2, pp. 304–308.
Rabinovich, Ya.I. and Derjaguin, B.V., Interaction of hydrophobized filaments in aqueous electrolyte solutions, Colloids Surf., 1988, vol. 30, pp. 243–251.
Rabinovich, Ya.I. and Yoon, R.H., Use of atomic force microscope for the measurements of hydrophobic forces, Colloids Surf., 1994, vol. 93, pp. 263–273.
Yoon, R.H., Flinn, D.H., and Rabinovich, Ya.I., Hydrophobic interactions between dissimilar surfaces, J. Colloid Interface Sci., 1997, vol. 185, pp. 363–370.
Rabinovich, Ya.I. and Yoon, R.H., Use of atomic force microscope for the measurements of hydrophobic forces between silanated silica plate and glass sphere, Langmuir, 1994, vol. 10, no. 6, pp. 1903–1909.
Churaev, N.V., Surface forces and physicochemistry of surface phenomena, Usp. Khim., 2004, vol. 73, no. 1, pp. 26–38.
Christenson, H.K. and Claesson, P.M., Direct measurements of the force between hydrophobic surfaces in water, Adv. Colloid Interface Sci., 2001, vol. 91, no. 3, pp. 391–436.
Hough, B. and Rendall, H.M., Adsorption of ionic surfactants, in Adsorption from Solutions on the Solid/Liquid Interface, Parfitt, G. and Rochester, C., Eds., London: Academic, 1983. Translated under the title Adsorbtsiya iz rastvorov na poverkhnosti tverdykh tel, Moscow: Mir, 1986, pp. 289–367.
Bitting, D., Effects of counterions on surfactants surface aggregates at the alumine/aqueouss solution interface, Langmuir, 1987, vol. 3, nos. 4–5, pp. 500–511.
Bijsterboch, B.N., Characterization of silica surface by adsorption of cationic surfactants, J. Colloid Interface Sci., 1974, vol. 47, no. 1, pp. 186–198.
Muller, V.M., Sergeeva, I.P., and Churaev, N.V., Adsorption of ionic surfactants on the charged surface: Two models, Kolloidn. Zh., 1995, vol. 57, no. 3, pp. 368–371.
Muller, V.M., Zakharova, M.A., Sobolev, V.D., and Churaev, N.V., Adsorption of CTAB from aqueous solutions on fused silica, Kolloidn. Zh., 1995, vol. 57, no. 3, pp. 400–406.
Clunie, J.S. and Ingram, B.T., Adsorption of nonionic surfactants, in Adsorption from Solutions on the Solid/Liquid Interface, Parfitt, G. and Rochester, C., Eds., London: Academic, 1983. Translated under the title Adsorbtsiya iz rastvorov na poverkhnosti tverdykh tel, Moscow: Mir, 1986, pp. 127–181.
Klimenko, N.A., Influence of micelle formation in aqueous solutions on the adsorption of oxyethylated nonionic surfactants in the carbon non-porous adsorbent (acetylene soot), Kolloidn. Zh., 1980, vol. 42, no. 3, pp. 561–566.
Mukerjee, R. and Mysels, K.J., Critical Micelle Concentrations of Aqueous Surfactant Systems, Washington: U.S. National Bureau of Standards, 1971.
Shinoda, K., Nakagawa, T., Tamamusi, B., and T. Isemura, T., Colloidal Surfactants, New York: Academic, 1963. Translated under the title Kolloidye poverkhnostno-aktivnye veshchestva, Moscow: Mir, 1966.
Koganovskii, A.M., Levchenko, T.M., and Kirichenko, V.A., Adsorbtsiya rastvorennykh veshchestv, (Adsorption of Dissolved Substances), Kiev: Naukova Dumka, 1977.
Koganovskii, A.M. and Klimenko, N.A., Fizikokhimicheskie osnovy izvlecheniya poverkhnostnoaktivnykh veshchestv iz vodnykh rastvorov i stochnykh vod (Physico-Chemical Fundamentals of the Extraction of Surfactants from Aqueous Solutions and Wastewater), Kiev: Naukova Dumka, 1978.
Pilgrimm, H., Aerosil-electrolyt-phasengrenze ohne anwesenheit vonbasenkationen, Colloid Polym. Sci., 1981, vol. 259, no. 6, pp. 1111–1115.
Sonntag, H., Itschenskij, V., and Koleznikova, R., Electrochemical characterization of aerosil OX-50 dispersions, Croat. Chem. Acta, 1987, vol. 60, no. 3, pp. 383–393.
Ermakova, L.E., Sidorova, M.P., Bogdanova, N.F., and Borisova, M.N., Electrochemical characteristics of silicon and titanium oxides and titanium-containing oxygen nanostructures on silica substrates, Colloid J., 1999, vol. 61, no. 6, pp. 714–718.
Gun’ko, V.M., Zarko, V.I., Leboda, R., and Chibowski, E., Aqueous suspensions of fumed oxides: Particle size distribution and zeta potential, Adv. Colloid Interface Sci., 2001, vol. 91, no. 1, pp. 1–112.
Sergacheva, I.P., Ermakova, T.B., Sobolev, V.D., and Churaev, N.V., Strukt. Din. Mol. Sist., 2003, issue X, part 3, pp. 49–52.
Vinogradova, O.I., On the attachment of particles of different degrees of hydrophobicity to a bubble in the collision, Kolloidn. Zh., 1993, vol. 55, no. 4, pp. 21–29.
Yakubov, G.E., Butt, H.J., and Vinogradova, O.I., Interaction forces between hydrophobic surfaces. attractive jump as an indication of formation of “stable” submicrocavities, J. Phys. Chem. B, 2000, vol. 104, no. 15, pp. 3407–3410.
Koishi, T., Yasuoka, K., Ebisuzaki, T., Yoo, S., and Zeng, X.C., Large-scale molecular-dynamics simulation of nanoscale hydrophobic interaction and nanobubble formation, J. Chem. Phys., 2005, vol. 123, p. 204707.
Shutilov, V.A., Osnovy fiziki ul’trazvuka. Uchebnoe posobie, Leningrad: Leningrad State University, 1980. Translated under the title Fundamental Physics of Ultrasound, London: Gordon and Breach, 1988.
Bunkin, N.F. and Bunkin, F.V., Bubbstons: stable microscopic gas bubbles in very dilute electrolytic solutions, Sov. Phys. JETP, 1992, vol. 74, no. 2, pp. 271–278.
Muller, V.M., The theory of reversible coagulation, Colloid J., 1996, vol. 58, no. 5, pp. 598–611.
Ishida, N., Sakamoto, M., Miyahara, M., and Higashitani, K., Attraction between hydrophobic surfaces with and without gas phase, Langmuir, 2000, vol. 16, no. 13, pp. 5681–5687.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.V. Golikova, O.M. Ioganson, V.S. Grigor’ev, 2013, published in Fizika i Khimiya Stekla.
Rights and permissions
About this article
Cite this article
Golikova, E.V., Ioganson, O.M. & Grigor’ev, V.S. Destabilization of sol of aerosil OX-50 in solutions of surfactants. II. Aerosil OX-25 in solutions of cationic and nonionic surfactants. Glass Phys Chem 39, 301–307 (2013). https://doi.org/10.1134/S1087659613030085
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659613030085