Abstract
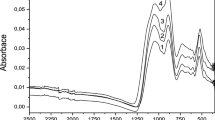
The concentration dependence of the electrical conductivity of glasses in the Tl2O-B2O3 system is studied. The nature of charge carriers in this system is experimentally investigated for the first time. It is demonstrated using the Hittorf, Tubandt, and Hebb-Liang-Wagner techniques and the Faraday law that neither Tl+ ions nor electrons are involved in the electricity transport. The verification of the Faraday law does not reveal the presence of thallium in the amalgam of the cathode or a change in the sample weight after electrolysis, to within the experimental error. This allows one to make the inference that protons can be charge carriers in glasses of the Tl2O-B2O3 system. It is shown using extended X-ray absorption fine structure (EXAFS) spectroscopy that Tl3+ ions and thallium Tl0 reduced to the metallic state are absent in the structure of the glasses under investigation. This means that thallium in glasses of the Tl2O-B2O3 system occurs only in the form of Tl+ ions. The analysis of the IR spectroscopic data leads to only a qualitative conclusion that the water content in the glasses insignificantly increases with an increase in the thallium oxide content. An increase in the electrical conductivity of glasses in the Tl2O-B2O3 system with an increase in the thallium oxide content is explained by the increase in the number of protons formed upon dissociation of H+[BO4/2]− structural-chemical units, because their concentration increases with increasing Tl2O content. In the structure of boron oxide, impurity hydrogen enters predominantly into the composition of H+[O2/2BO−] structural-chemical units, for which the dissociation energy is higher than that for the H+[BO4/2]− structural-chemical units. The increase in the concentration of H+[BO4/2]− structural-chemical units is accompanied by the increase in the number of dissociated protons, which are charge carriers in glasses of the Tl2O-B2O3 system.
Similar content being viewed by others
References
Zhabrev, V.A. and Svidorov, S.I., Ion Diffusion in Oxide Glasses and Melts: I. Bibliography, Fiz. Khim. Stekla, 2003, vol. 29, no. 2, pp. 210–229 [Glass Phys. Chem. (Engl. transl.), 2003. vol. 29, no. 2, pp. 140–159].
Ingram, M.D., Ionic Conductivity in Glass, Phys. Chem. Glasses, 1987, vol. 26, no. 6, pp. 215–234.
Sokolov, I.A., Murin, I.V., Naraev, V.N., and Pronkin, A.A., On the Nature of Current Carriers in Alkali-Free Glasses Based on Silicon, Boron, and Phosphorus Oxides, Fiz. Khim. Stekla, 1999, vol. 25, no. 5, pp. 593–613 [Glass Phys. Chem. (Engl. transl.), 1999, vol. 25, no. 5, pp. 454–468].
Naraev, V.N., The Influence of Water on the Glass Properties, Fiz. Khim. Stekla, 2004, vol. 30, no. 5, pp. 499–530 [Glass Phys. Chem. (Engl. transl.), 2004, vol. 30, no. 5, pp. 367–389].
Startsev, Yu.K. and Safutina, T.V., Influence of Residual Water in a Glass on Its Rheological and Relaxation Properties (by the Example of a 5Na2O-95B2O3 Glass), Fiz. Khim. Stekla, 2001, vol. 27, no. 5, pp. 601–610 [Glass Phys. Chem. (Engl. transl.), 2001, vol. 27, no. 5, pp. 411–417].
Startsev, Yu.K. and Golubeva, O.Yu., Specific Features of Changes in the Properties of One-and Two-Alkali Borate Glasses Containing Water: I. Viscosity, Thermal Expansion, and Kinetics of Structural Relaxation in Binary Alkali Borate Glasses, Fiz. Khim. Stekla, 2002, vol. 28, no. 3, pp. 230–245 [Glass Phys. Chem. (Engl. transl.), 2002, vol. 28, no. 3, pp. 159–169].
Sokolov, I.A., Naraev, V.N., Murin, I.V., Pronkin, A.A., and Naraev, A.V., Electrochemical Study of Glasses in the System Na2O-B2O3, Zh. Prikl. Khim. (S.-Peterburg), 2002, vol. 75, no. 8, pp. 1226–1233 [Russ. J. Appl. Chem. (Engl. transl.), 2002, vol. 75, no. 8, pp. 1240–1247].
Ostroumov, G., Determination of the Transport Numbers in Sodium Tetraborate Glasses, Zh. Obshch. Khim., 1949, vol. 19, no. 3, pp. 407–410.
Markin, B.N., Electrical Conductivity of Thallium Borate Glasses, Zh. Tekh. Fiz., 1958, vol. 22, no. 6, pp. 941–945.
MDL-SciGlass TM 5.0, San Leandro: MDL Information Systems, 2002.
Sokolov, I.A., Murin, I.V., and Pronkin, A.A., The Nature of Charge Carriers and Their Transport Numbers in Glasses of the Ag2O-B2O3 System, Fiz. Khim. Stekla, 2006, vol. 32, no. 1, pp. 90–99 [Glass Phys. Chem. (Engl. transl.), 2006, vol. 32, no. 1, pp. 63–69].
SciGlass 7.0, Premium Edition, ITC, 2008, http://www.sciglass.info.
Startsev, Yu.K., Technique for Measuring the Electric Conductivity of Glasses and Melts over a Wide Temperature Range Covering the Glass Transition Region, Fiz. Khim. Stekla, 2000, vol. 26, no. 1, pp. 103–115 [Glass Phys. Chem. (Engl. transl.), 2000, vol. 26, no. 1, pp. 73–82].
Hughes, K. and Isard, J.O., Ionic Transport in Glasses, in Physics of Electrolytes, Hladik, J., Ed., New York: Academic, 1972. Fizika elektrolitov. Protsessy perenosa v tverdykh elektrolitakh, Moscow: Mir, 1978, ch. 10.
Key, R.L., Measurement of Transport Numbers, in Techniques of Electrochemistry, Yeager, E. and Salkind, A.J., Eds., New York: Wiley, 1972. Translated under the title Metody izmereniya v elektrokhimii, Moscow: Mir, 1977.
Wagner, C., Galvanic Cells with Solid Electrolytes Involving Ionic and Electronic Conduction, Proceedings of the VII Meeting of the International Commission on Electrochemical, Thermal, and Kinetic Processes, London, 1955, London: Butterworth, 1957, pp. 361–389.
Liang, C., Determination of the Electronic Transference Numbers of Solid Electrolytes, Trans. Faraday Soc., 1970, vol. 65, no. 564, pp. 3369–3374.
Franz, H., Solubility of Water in Alkali Borate Glasses, J. Am. Ceram. Soc., 1966, vol. 49, no. 9, pp. 473–477.
Eagan, R. V. and Bergeron, C.G., Determination of Water in Lead Borate Glasses, J. Am. Ceram. Soc., 1972, vol. 55, no. 1, pp. 53–54.
Merker, L. and Scholze, H., Der Einflüß des Wassergehaltes von Silikatglässern auf Transformations-und Erweichngsverhalten, Glastech. Ber., 1962, vol. 35, no. 1, pp. 37–43.
Adams, R.V., Infrared Absorption Due to Water in Glasses, Phys. Chem. Glasses, 1961, vol. 2, no. 2, pp. 39–49.
Bartolomew, R.F., Water in Glass, in Treatise on Materials Science and Technology, Doremus, R.H. and Tomozawa, M., Eds., New York: Academic, 1982, vol. 22, pp. 75–127.
Bouaziz, R. and Touboul, M., Le systeme binaire monoborate de thallium-anhydride borique, C. R. Acad. Sci., Ser. C, 1967, vol. 264, no. 16, pp. 1374–1377.
Myuller, R.L., Elektroprovodnost’ stekloobraznykh veshchestv. Sbornik trudov (Electrical Conductivity of Vitreous Compounds: A Collection of Articles), Leningrad: Leningr. Gos. Univ., 1966 [in Russian].
Bray, P.J. and O’Keete, Y.G., Nuclear Magnetic Resonance Investigation of the Structure of Borate Glasses, J. Non-Cryst. Solids, 1980, vols. 38–39, pp. 93–98.
Greenblatt, S. and Bray, P.J., A Discussion of the Fraction of Four-Co-Ordinated Boron Atoms Present in Borate Glasses, Phys. Chem. Glasses, 1967, vol. 8, no. 6, pp. 213–217.
Myuller, R.L., The Solid State Chemistry and the Vitreous State, in Khimiya tverdogo tela (The Solid State Chemistry), Leningrad: Leningr. Gos. Univ., 1965, pp. 9–63 [in Russian].
Bashilova, N.I., Thallium, in Kratkaya khimicheskaya entsiklopediya (Concise Chemical Encyclopedia), Moscow: Sovetskaya Entsiklopediya, 1967, vol. 5, pp. 10–18 [in Russian].
Ernsberger, F.M., Molecular Water in Glass, J. Am. Ceram. Soc., 1977, vol. 60, nos. 1–2, pp. 91–92.
Sakka, S., Kamiva, R., and Huang, Z.J., Effects of a Small Amount of Water on Characteristics of Glasses, Res. Rep. Fac. Eng. Mie Univ., 1982, vol. 7, pp. 137–159.
Tomozawa, M., Water in Glass, J. Non-Cryst. Solids, 1985, vol. 73, nos. 1–3, pp. 197–204.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © I.A. Sokolov, I.V. Murin, V.D. Khripun, N.A. Valova, Yu.K. Startsev, A.A. Pronkin, 2008, published in Fizika i Khimiya Stekla.
Rights and permissions
About this article
Cite this article
Sokolov, I.A., Murin, I.V., Khripun, V.D. et al. Electrical conductivity and the nature of charge carriers in glasses of the Tl2O-B2O3 system. Glass Phys Chem 34, 227–239 (2008). https://doi.org/10.1134/S1087659608030012
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659608030012