Abstract
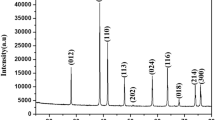
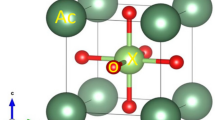
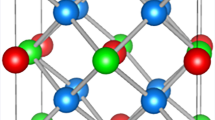
The structure of the mixed oxides Me x (Ce0.5Zr0.5)1−x O y (x = 0, 0.1–0.30; y < 2) doped with trivalent Gd and Pr metals is investigated using the X-ray powder diffraction analysis, the full-profile analysis of the diffraction pattern, and EXAFS spectroscopy. The mixed oxides are solid solutions with a structure similar to the CeO2 structure. An increase in the content of the introduced trivalent metal brings about an increase in the unit cell parameter and a disordering of the structure of the oxides under investigation.
Similar content being viewed by others
References
Logan, D. and Shelef, M., Oxygen Availability in Mixed Cerium/Praseodymium Oxides and the Effect of Noble Metals, J. Mater. Res., 1994, vol. 9, no. 2, pp. 468–475.
Takasu, Y., Sugino, T., and Matsuda, Y., Electrical Conductivity of Praseodymia-Doped Ceria, J. Appl. Electrochem., 1984, vol. 14, no. 1, pp. 79–81.
Nauer, M., Ftikos, C., and Steele, B.C.H., An Evaluation of Ce-Pr Oxides and Ce-Pr-Nb Oxide Mixed Conductors for Cathodes of Solid Oxide Fuel Cells: Structure, Thermal Expansion, and Electrical Conductivity, J. Eur. Ceram. Soc., 1994, vol. 14, no. 6, pp. 493–499.
Kaspar, J., Fornasiero, P., and Graziani, M., Use of CeO2-Based Oxides in the Three-Way Catalysis, Catal. Today, 1999, vol. 50, no. 2, pp. 285–298.
Nigge, U., Wiemhöfer, H.-D., Römer, E.W.J., Bouwmeester, H.J.M., and Schulte, T.R., Composites of Ce0.8Gd0.2O1.9 and Gd0.7Ca0.3CoO3−δ as Oxygen Permeable Membranes for Exhaust Gas Sensors, Solid State Ionics, 2002, vol. 146, nos. 1–2, pp. 163–164.
Otsuka, K., Sunada, E., Ushiyama, T., and Yamanaka, I., The Production of Synthesis Gas by Redox of Cerium Oxide, Stud. Surf. Sci. Catal., 1997, vol. 107, pp. 531–536.
Trovarelli, A., Catalytic Properties of Ceria and CeO2-Containing Materials, Catal. Rev.—Sci. Eng., 1996, vol. 38, no. 4, pp. 439–520.
Fornasiero, P., Balducci, G., Di Monte, R., and Kašpar, J., Modification of the Redox Behaviour of CeO2 Induced by Structural Doping with ZrO2, J. Catal., 1996, vol. 164, no. 1, pp. 173–183.
Pechini, M.P., Method of Preparing Lead and Alkaline-Earth Titanates and Niobates and Coating Methods Using the Same to Form a Capacitor, US Patent 3330697, 1967.
Kuznetsova, T.G., Sadykov, V.A., Moroz, E.M., Trukhan, S.N., Paukshtis, E.A., Kolomiichuk, V.N., Burgina, E.B., Zaikovskii, V.I., Fedotov, M.A., Lunin, V.V., and Kemnitz, E., Preparation of Ce-Zr-O Composites by a Polymerized Complex Method, Stud. Surf. Sci. Catal., 2002, vol. 143, pp. 659–667.
Kochubei, D.I., Methods for Extracting Structural Information from EXAFS Spectra, in Rentgenospektral’nyi metod izucheniya struktury amorfnykh tel. EXAFS-spektroskopiya (X-ray Spectroscopic Method for Investigation of the Structure of Amorphous Materials: EXAFS Spectroscopy), Novosibirsk: Nauka, 1988, pp. 171–213 [in Russian].
Binsted, N., Campbell, J.V., Gurman, S.J., and Stephenson, P.C., SERC Daresbury Laboratory EXCURV92 Program, Warrington, Cheshire (United Kingdom): Daresbury Laboratory, 1991.
Mamontov, E. and Egami, T., Lattice Defects and Oxygen Storage Capacity of Nanocrystalline Ceria and Ceria-Zirconia, J. Phys. Chem. B, 2000, vol. 104, no. 47, pp. 11110–11116.
Mamontov, E., Brezny, R., Koranne, M., and Egami, T., Nanoscale Heterogeneities and Oxygen Storage Capacity of Ce0.5Zr0.5O2, J. Phys. Chem. B, 2003, vol. 107, no. 47, pp. 13007–13014.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.P. Kol’ko, D.A. Zyuzin, V.A. Sadykov, V.V. Kriventsov, E.M. Moroz, 2007, published in Fizika i Khimiya Stekla.
Rights and permissions
About this article
Cite this article
Kol’ko, V.P., Zyuzin, D.A., Sadykov, V.A. et al. Structure of the mixed oxides Me x (Ce0.5Zr0.5)1−x O y (Me = Gd, Pr). Glass Phys Chem 33, 335–339 (2007). https://doi.org/10.1134/S1087659607040050
Issue Date:
DOI: https://doi.org/10.1134/S1087659607040050