Abstract
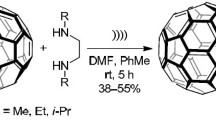
Morpholine–fullerene C60 monoadducts were synthesized for the first time by reaction of C60 with biogenic amines (noradrenaline and adrenaline) under ultrasonic irradiation in toluene/DMF at room temperature on exposure to air. The key intermediate in the synthesis of the C60–adrenaline cycloadduct, C60 radical anion generated by single-electron transfer from the adrenaline molecule to C60, was detected by ESR (C60‒·, g = 2.0000, ΔH1/2 = 3.2 G).

Similar content being viewed by others
REFERENCES
Zieleniewska, A., Lodermeyer, F., Roth, A., and Guldi, D.M., Chem. Soc. Rev., 2018, vol. 47, p. 702. https://doi.org/10.1039/C7CS00728K
Jia, L., Chen, M., and Yang, S., Mater. Chem. Front., 2020, vol. 4, p. 2256. https://doi.org/10.1039/D0QM00295J
Nakamura, E. and Isobe, H., Acc. Chem. Res., 2003, vol. 36, p. 807. https://doi.org/10.1021/ar030027y
Castro, E., Garcia, A.H., Zavala, G., and Echegoyen, L., J. Mater. Chem. B, 2017, vol. 5, p. 6523. https://doi.org/10.1039/C7TB00855D
Anilkumar, P., Lu, F., Cao, L., Luo, P.G., Liu, J.-H., Sahu, S., Tackett, K.N. II, Wang, Y., and Sun, Y.-P., Curr. Med. Chem., 2011, vol. 18, p. 2045. https://doi.org/10.2174/092986711795656225
Li, F.-B., Liu, T.-X., and Wang, G.-W., J. Org. Chem., 2008, vol. 73, p. 6417. https://doi.org/10.1021/jo8007868
Yang, H.-T., Liang, X.-C., Wang, Y.-H., Yang, Y., Sun, X.-Q., and Miao, C.-B., Org. Lett., 2013, vol. 15, p. 4650. https://doi.org/10.1021/jo402079m
Zhang, X.-F., Li, F.-B., Shi, J.-L., Wu, J., and Liu, L., New J. Chem., 2016, vol. 40, p. 1626. https://doi.org/10.1039/C5NJ02503F
Takeda, Y., Enokijima, S., Nagamachi, T., Nakayama, K., and Minakata, S., Asian J. Org. Chem., 2013, vol. 2, p. 91. https://doi.org/10.1002/ajoc.201200114
Yang, H.-T., Ren, W.-L., Dong, C.-P., Yang, Y., Sun, X.-Q., and Miao, C.-B., Tetrahedron Lett., 2013, vol. 54, p. 6799. https://doi.org/10.1016/j.tetlet.2013.09.002
Yang, H.-T., Xing, M.-L., Zhu, Y.-F., Sun, X.-Q., Cheng, J., Miao, C.-B., and Li, F.-B., J. Org. Chem., 2014, vol. 79, p. 1487. https://doi.org/10.1021/jo4025573
You, X. and Wang, G.-W., J. Org. Chem., 2014, vol. 79, p. 117. https://doi.org/10.1021/jo402354w
Zhen, J., Liu, Q., Chen, X., Li, D., Qiao, Q., Lu, Y., and Yang, S., J. Mater. Chem. A, 2016, vol. 4, p. 8072. https://doi.org/10.1039/C6TA02016J
Dzhemilev, U.M., Ibragimov, A.G., Tuktarov, A.R., Pudas, M., and Valyamova, F.G., RU Patent no. 2309938C1, 2007.
Liu, Q., Zhen, J., Zhou, W., Chen, X., Li, D., and Yang, S., Org. Electron., 2016, vol. 39, p. 191. https://doi.org/10.1016/j.orgel.2016.10.009
Yang, H.-T., Ge, J., Lu, X.-W., Sun, X.-Q., and Miao, C.-B., J. Org. Chem., 2017, vol. 82, p. 5873. https://doi.org/10.1021/acs.joc.7b00741
Kinzyabaeva, Z.S. and Sharipov, G.L., Ultrason. Sonochem., 2018, vol. 42, p. 119. https://doi.org/10.1016/j.ultsonch.2017.11.012
Kinzyabaeva, Z.S. and Sharipov, G.L., Russ. J. Org. Chem., 2018, vol. 54, p. 1112. https://doi.org/10.1134/S1070428018070254
Kinzyabaeva, Z.S., Dmitriev, A.M., and Sabirov, D.Sh., Fullerenes, Nanotubes, Carbon Nanostruct., 2021, vol. 29, p. 601. https://doi.org/10.1080/1536383X.2021.1873782
Kinzyabaeva, Z.S., Chem. Heterocycl. Compd., 2021, vol. 57, no. 5, p. 602. https://doi.org/10.1007/s10593-021-02950-2
Isaacs, L., Wehrsig, A., and Diederich, F., Helv. Chim. Acta, 1993, vol. 76, p. 1231. https://doi.org/10.1002/hlca.19930760310
Elemes, Y., Silverman, S.K., Sheu, C., Kao, M., Foote, C.S., Alvarez, M.M., and Whetten, R.L., Angew. Chem., Int. Ed. Engl., 1992, vol. 31, p. 351. https://doi.org/10.1002/anie.199203511
Kinzyabaeva, Z.S., Sadykov, R.A., and Sharipov, G.L., Fullerenes, Nanotubes, Carbon Nanostruct., 2019, vol. 27, p. 878. https://doi.org/10.1080/1536383X.2019.1653857
Hirsch, A., Li, Q., and Wudl, F., Angew. Chem., Int. Ed. Engl., 1991, vol. 30, p. 1309. https://doi.org/10.1002/anie.199113091
Wudl, F., Hirsch, A., Khemani, K.C., Suzuki, T., Allemand, P.-M., Koch, A., Eckert, H., Srdanov, G., and Webb, H.M., Fullerenes. Synthesis, Properties, and Chemistry of Large Carbon Clusters, ACS Symposium Series vol. 481, Hammond, G.S. and Kuck, V.J., Eds., Washington: American Chemical Society, 1992, p. 161.
Lobach, A.S., Goldshleger, N.F., Kaplunov, M.G., and Kulikov, A.V., Chem. Phys. Lett., 1995, vol. 243, p. 22. https://doi.org/10.1016/0009-2614(95)00811-H
ACKNOWLEDGMENTS
Structural studies were performed at the Agidel joint center, Institute of Petrochemistry and Catalysis, Ufa Federal Research Center, Russian Academy of Sciences. The ESR spectra were recorded at the Spectrum joint center, Institute of Molecular and Crystal Physics, Ufa Federal Research Center, Russian Academy of Sciences.
Funding
This study was financially supported in the framework of state assignment no. FMRS-2022-0077.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare the absence of conflict of interest.
Rights and permissions
About this article
Cite this article
Kinzyabaeva, Z.S., Sabirov, D.S. Synthesis of Fullerene C60 Hybrids with Catecholamines under Ultrasonic Irradiation. Russ J Org Chem 58, 1915–1919 (2022). https://doi.org/10.1134/S1070428022120223
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428022120223